Intravenous drug access system
a drug access system and intravenous technology, applied in the field of fluid transfer devices, can solve the problems of affecting the health of patients, preventing the flow of primary and secondary fluids to the patient, and spiking connectors not allowing fluid, etc., to achieve convenient and controlled fluid flow, high rejection rate, and easy penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
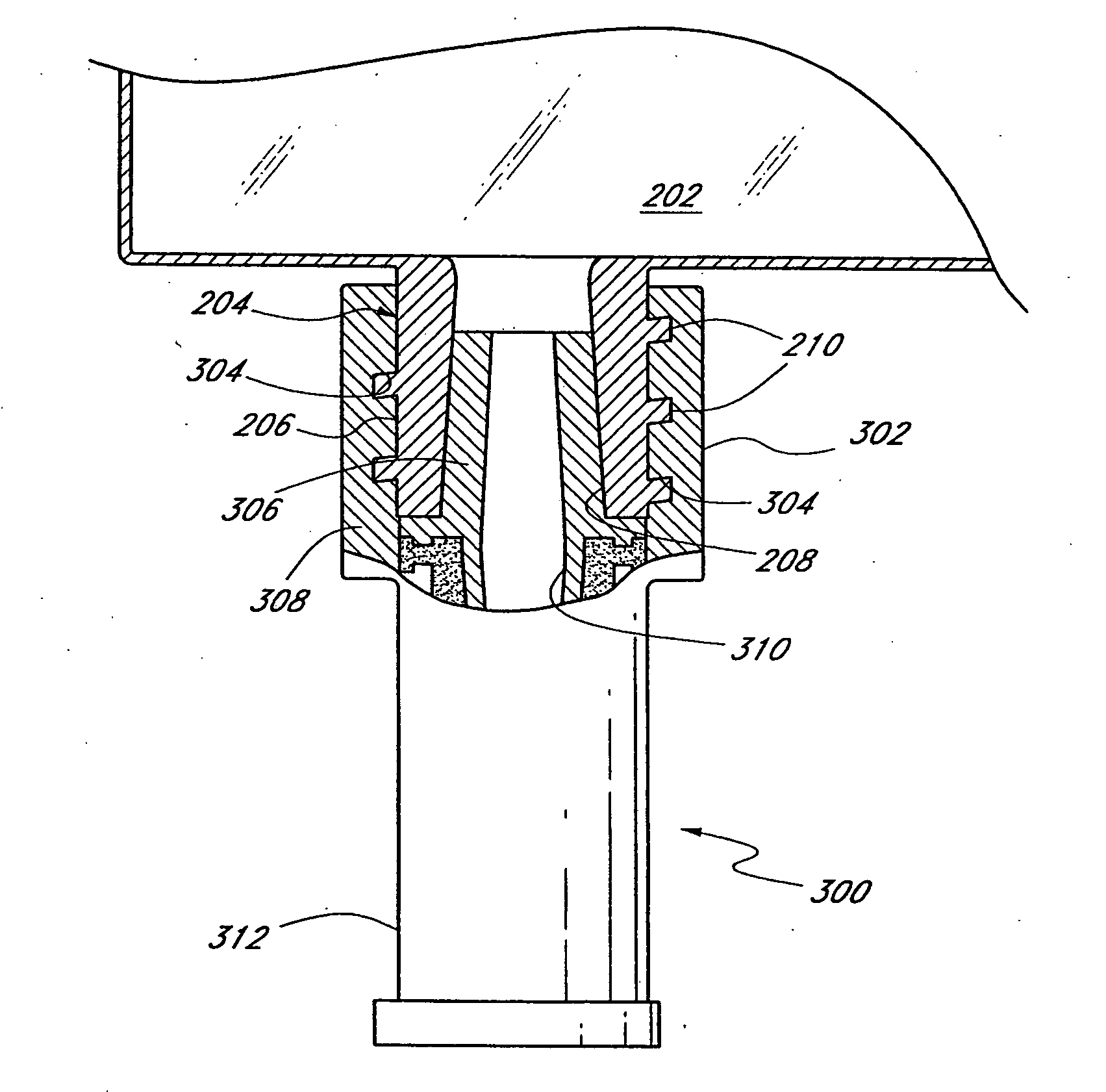
[0026] As discussed above, FIG. 1 shows a conventional IV bag having inlet and outlet ports for the transmission of fluids into the IV bag and to the patient, respectively. FIG. 2 illustrates the drug access system 200 of the present invention, which is an improvement over the prior art IV bag. The inventive drug access system 200 comprises a collapsible container 202, such as an IV bag, that includes at least one integral inlet (injection) port 204 and at least one integral outlet port (not shown). The inlet port 204 comprises a housing 206 made of, e.g., a hard durable plastic, metal, or any other material known to those of skill in the art. The port 204 defines an internal conduit 208 for allowing fluid communication between the IV bag 202 and a discrete connector or valve. The housing 206 has a female fitting at a distal end, commonly referred to as a female luer, for acceptance of a male luer therein. Preferably, the housing 206 includes external threads 210 for mating with a m...
second embodiment
[0031] With reference to FIGS. 4 and 5, the invention comprises a connector 500 for providing fluid communication between a medical connector or valve 600 and an IV bag 402 having a conventional sealed inlet (injection) port 404. The inlet port 404 of the IV bag 402 includes a conventional septum 406 within the interior of the inlet port 404 to prevent the flow of fluid out of the IV bag 402. An annular locking member 408 extends radially from a midsection of the inlet port 404. The annular locking member 408 may be used to lock a valve or connector to the inlet port 404, as described in further detail below.
[0032] Preferably, the medical connector 500 includes a main housing 514 that is preferably integrally molded from a suitable plastic material, such as polycarbonate, although other medically inert materials may be used. The housing 514 defines an internal fluid conduit 518 having a first, proximal end with a spike or puncture member 526. The spike 526 is configured for piercing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com