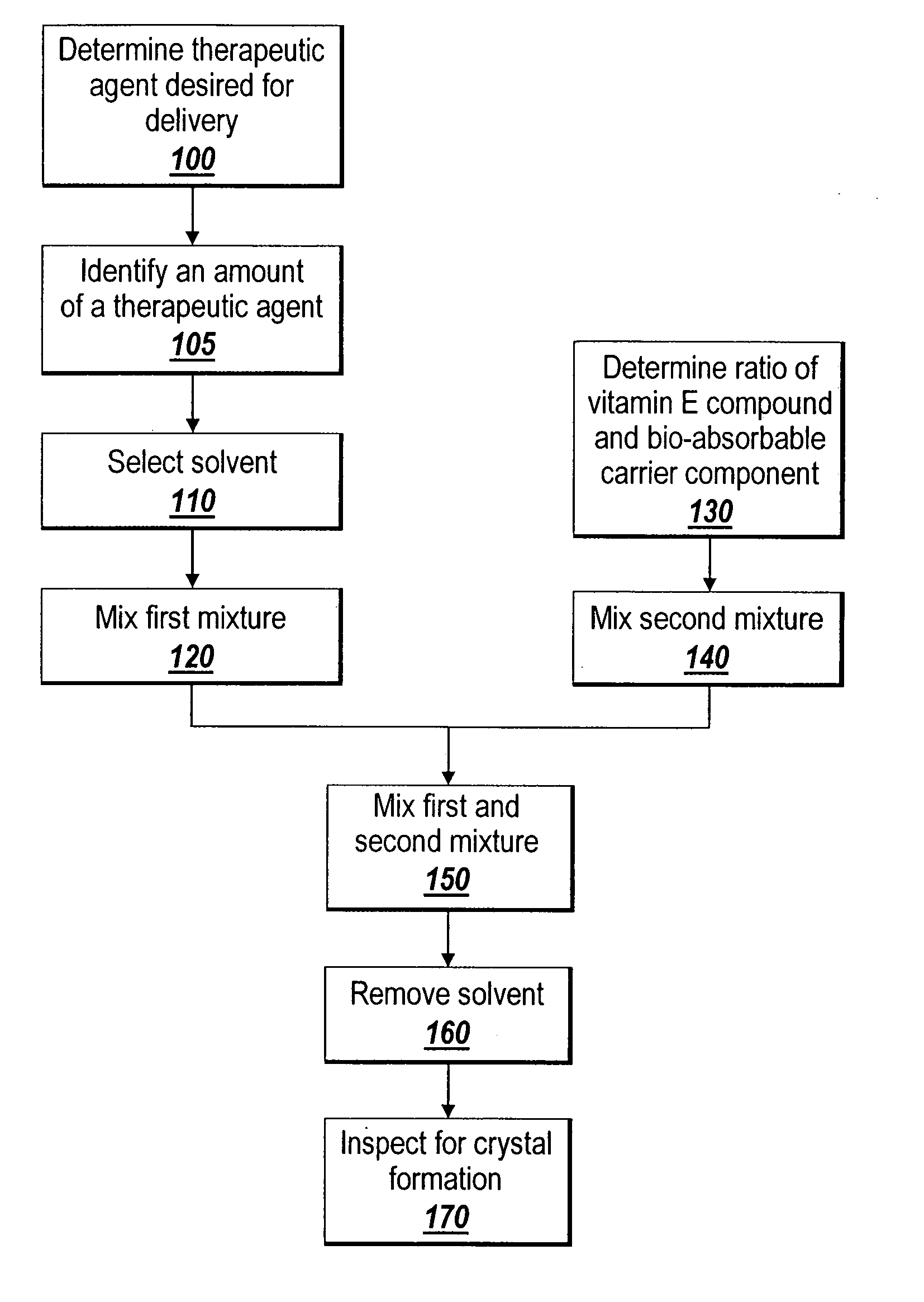
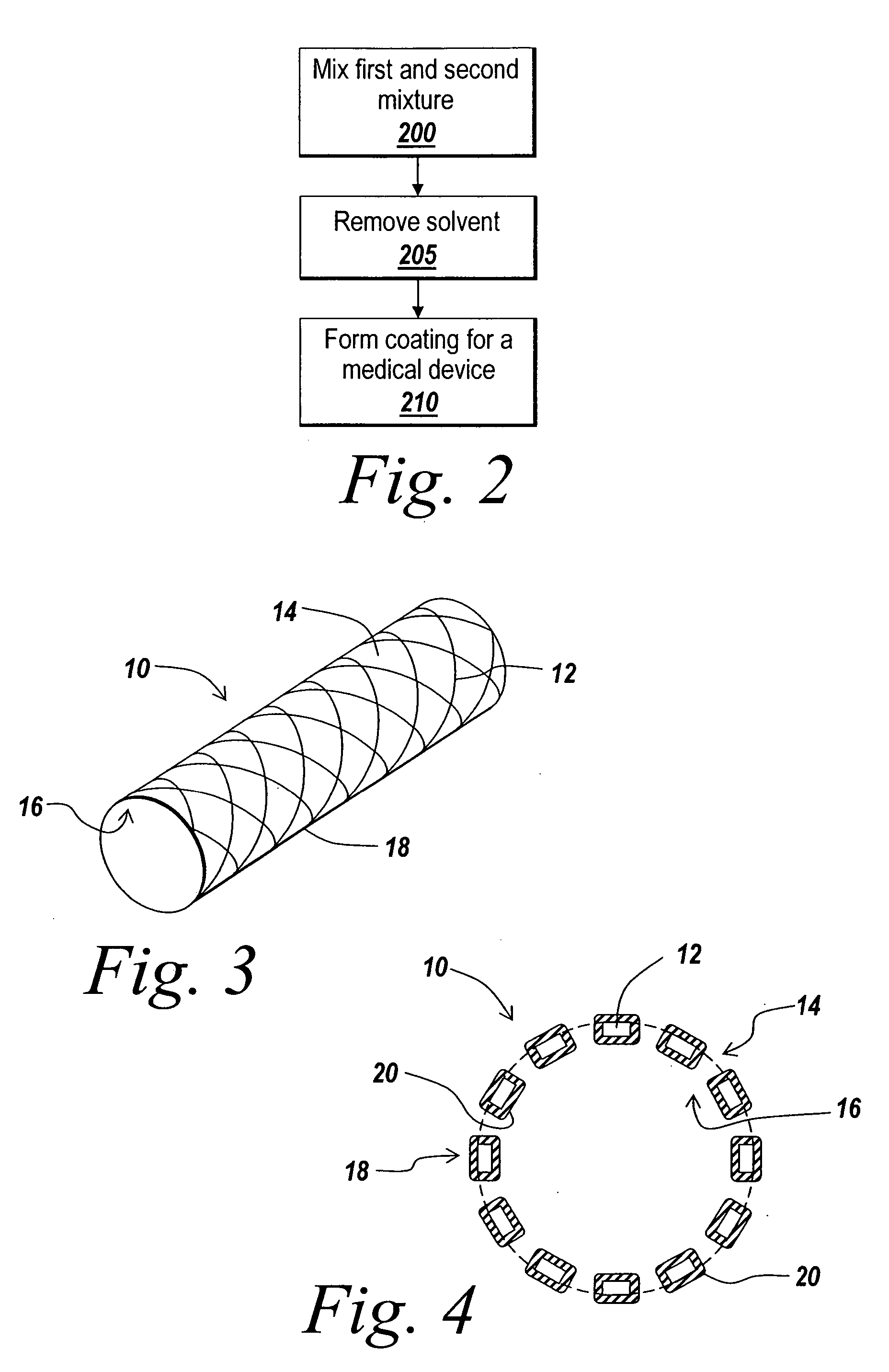
Solubilizing a drug for use in a coating
a drug and coating technology, applied in the field of coatings and preparations of coatings, can solve the problems of serious bleeding complications and other side effects, orally administered drugs may not achieve the desired effect in the area of the body, and achieve the effects of improving consistency and conformability, enhancing adhesion of coatings, and increasing viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example # 1
EXAMPLE #1
[0119] A bio-absorbable carrier component in combination with a vitamin E compound was made by mixing 1.5 grams of vitamin E and 3.5 grams of fish oil to form a base coating (30% vitamin E). A sample was then prepared by first dissolving 28 mg of rapamycin in 529 mg of NMP (N-methyl-2-pyrrolidone). After the drug was fully dissolved in the solvent, 502 mg of the 30% vitamin E / 70% fish oil base coat was added and the solution was vortexed until thoroughly mixed. A drop of the coating was then placed on a microscope slide and the sample was dried over night under vacuum in a bell jar. This was the maximum level of drug loading (5.3%) attainable with this formulation before crystals began to form after drying. A bio-absorbable carrier component in combination with a vitamin E compound was then made by mixing 3.5 grams of vitamin E and 1.5 grams of fish oil to form a base coating (70% vitamin E). A sample was then prepared by first dissolving 110 mg of rapamycin in 244 mg of N...
example # 2
EXAMPLE #2
[0120] A bio-absorbable carrier component in combination with a vitamin E compound was made by mixing 3.5 grams of Vitamin E and 1.5 grams of fish oil to form a base coating (70% vitamin E / 30% fish oil). A sample was then prepared by first dissolving 41 mg of melatonin in 270 mg of NMP (N-methyl-2-pyrrolidone). After the drug was fully dissolved in the solvent, 316 mg of the 70% vitamin E / 30% fish oil base coat was added and the solution was vortexed until thoroughly mixed. A drop of the coating was then placed on a microscope slide and the sample was dried over night under vacuum in a bell jar. This was the maximum level of drug loading (11.5%) attainable with this formulation before crystals began to form after drying. A bio-absorbable carrier component in combination with a vitamin E compound was then made by mixing 3.5 grams of vitamin E and 1.5 grams of fish oil fatty acids (FOFA) to form a base coating (70% vitamin E / 30% FOFA). A sample was then prepared by first dis...
example # 3
EXAMPLE #3
[0121] A bio-absorbable carrier component in combination with a vitamin E compound was made by mixing 3.5 grams of vitamin E and 1.5 grams of fish oil to form a base coating (70% vitamin E / 30% fish oil). A sample was then prepared by first dissolving 8.4 mg of paclitaxel in 153 mg of ethanol. After the drug was fully dissolved in the solvent, 162.4 mg of the 70% vitamin E / 30% fish oil base coat was added and the solution was vortexed until thoroughly mixed. A drop of the coating was then placed on a microscope slide and the sample was dried over night under vacuum in a bell jar. This was the maximum level of drug loading (4.9%) attainable with this formulation before crystals began to form after drying. A bio-absorbable carrier component in combination with a vitamin E compound was then made by mixing 3.5 grams of vitamin E and 1.5 grams of fish oil to form a base coating (70% vitamin E / 30% fish oil). A sample was then prepared by first dissolving 8.4 mg of paclitaxel in 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| bio-absorbable | aaaaa | aaaaa |
| bio-absorbable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com