Cerebral spinal fluid flow sensing device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
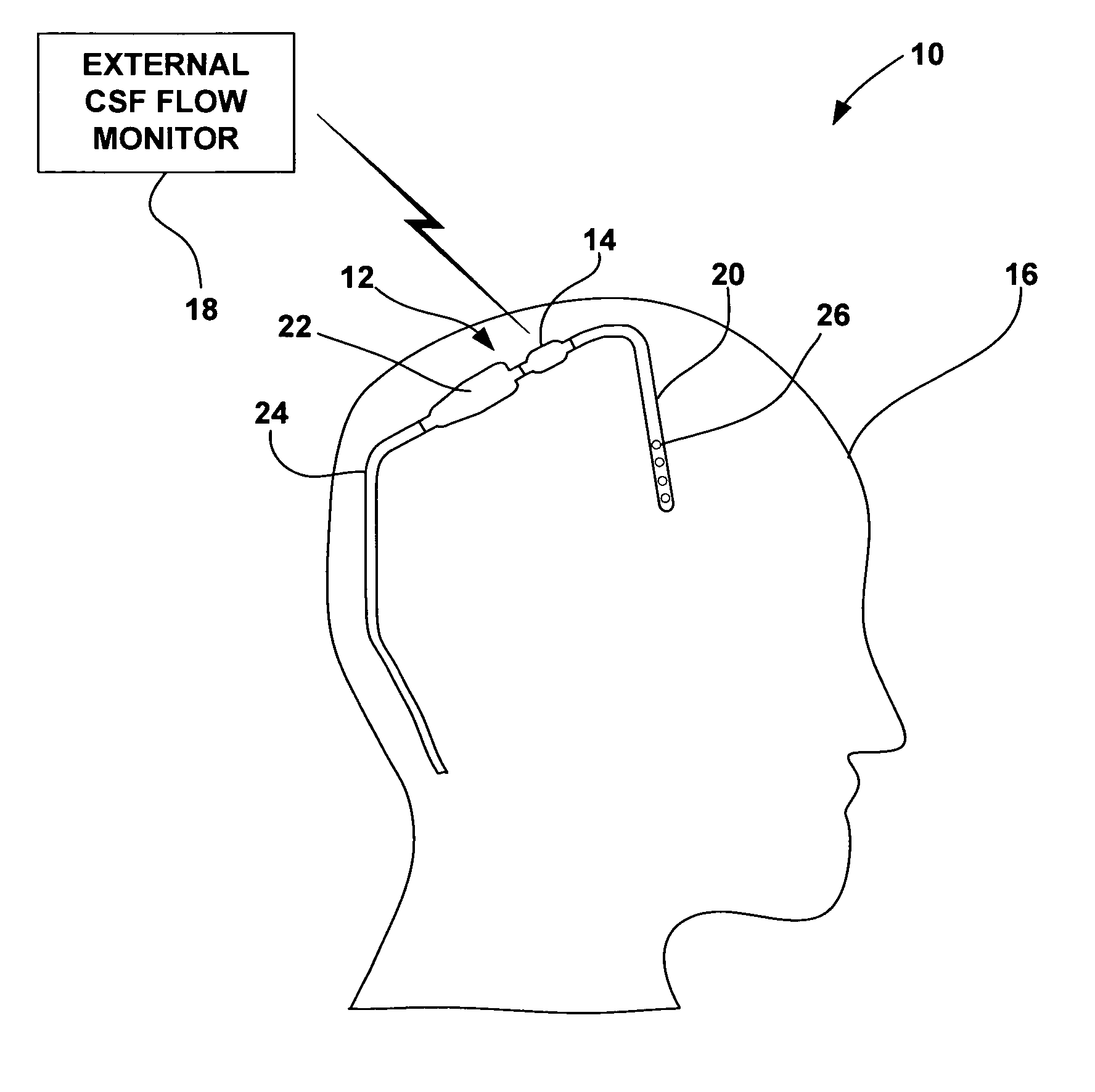
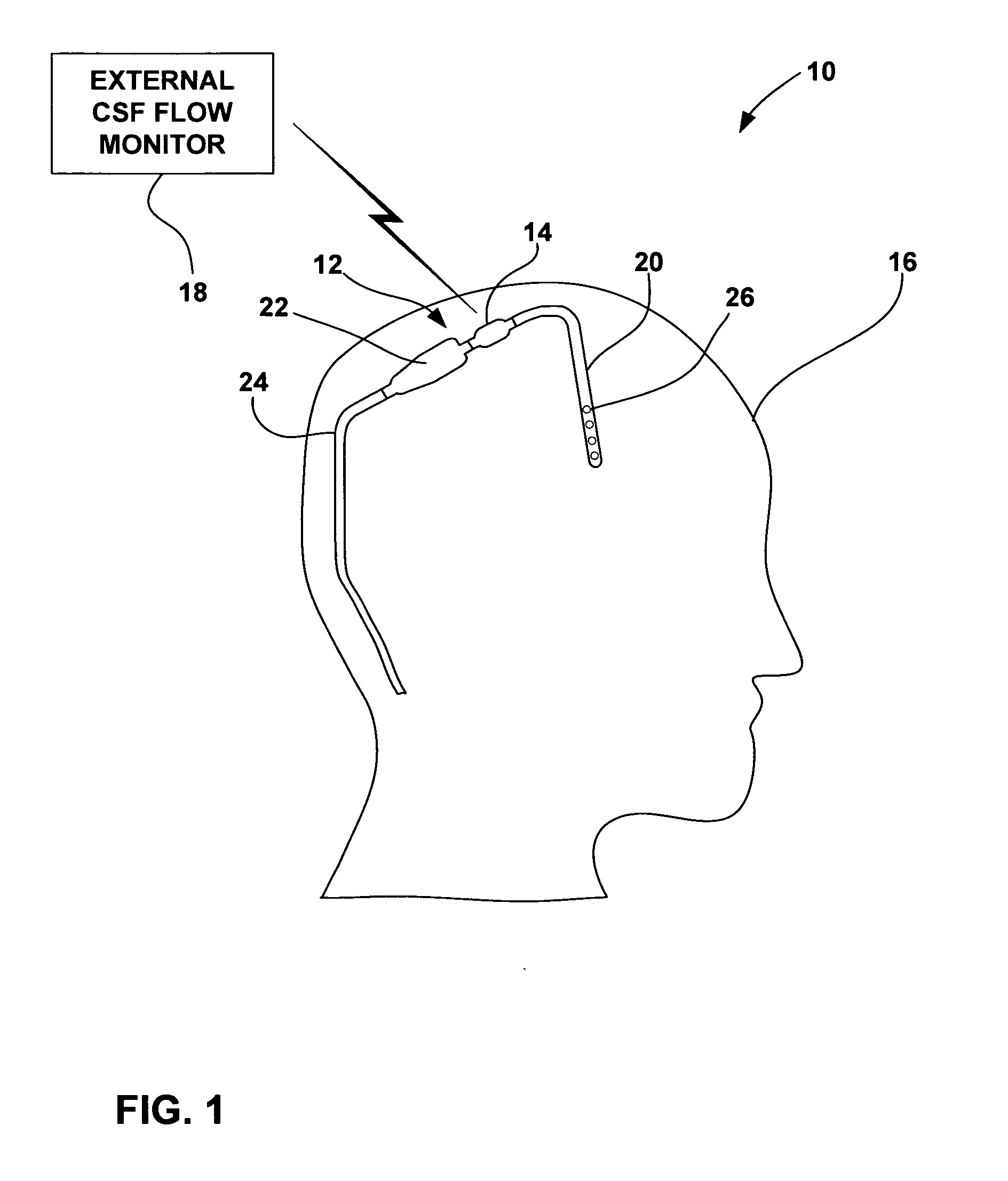
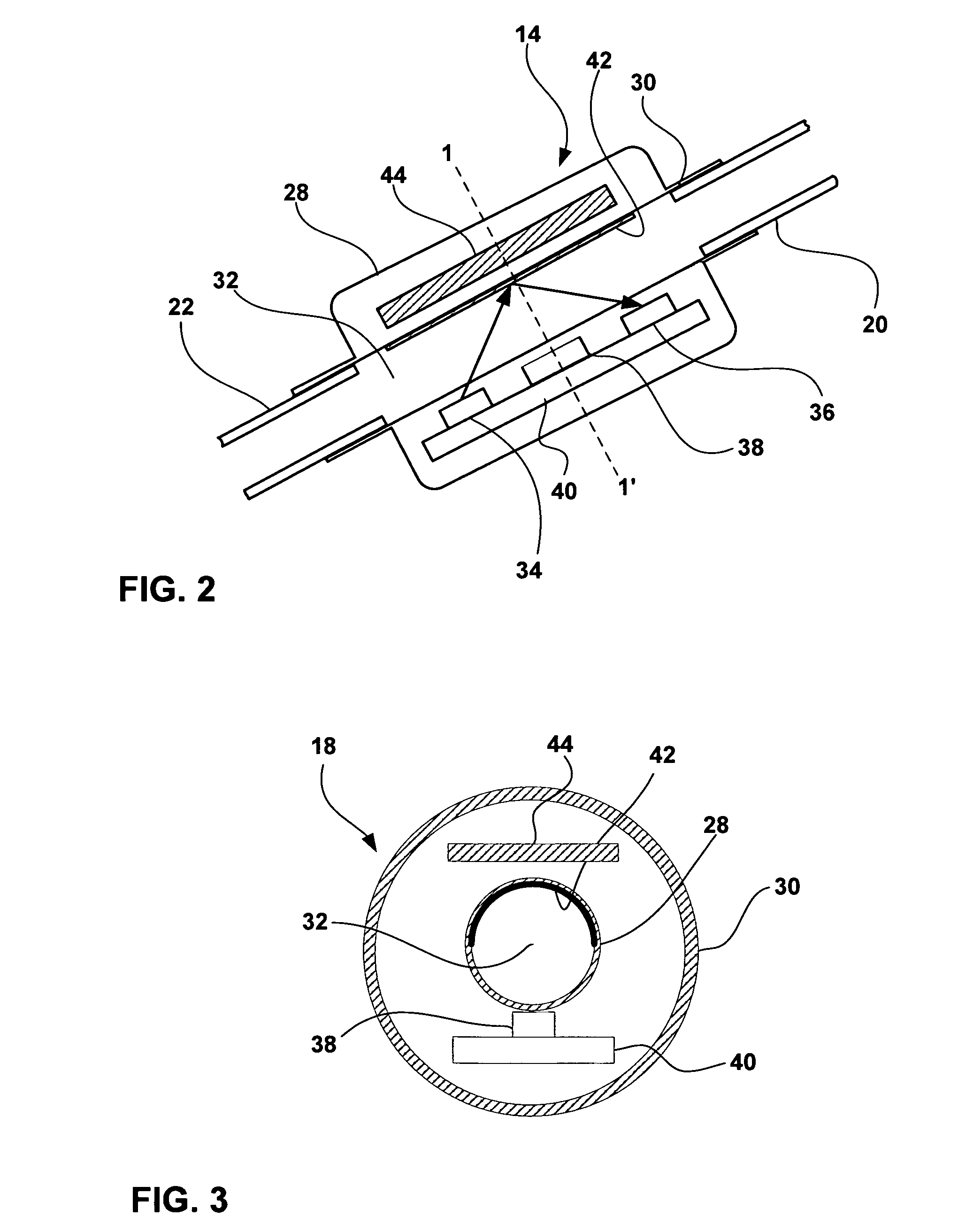
[0022]FIG. 1 is a schematic diagram illustrating a CSF flow monitoring system 10 having an implantable ventricular shunt 12 and a CSF flow sensor 14 in conjunction with a patient 16. CSF flow sensor 14 transmits CSF flow information to an external CSF flow monitor 18 via wireless telemetry. As shown in FIG. 1, shunt 12 is implanted within the cranium of patient 16, and includes a ventricular catheter 20, a CSF flow control valve 22 and a drainage catheter 24. Ventricular catheter 20, flow control valve 22 and drainage catheter 24 define a CSF flow path within shunt 12.
[0023] Ventricular catheter 20 may be bent and angled downward to extend into a brain ventricle. Alternatively, ventricular catheter 20 may be substantially straight, but couple to an angled coupling joint extending from control valve 22. Ventricular catheter 20 may define a plurality of holes 26 to receive CSF from a brain ventricle. Shunt 12 may be, for example, a ventriculoperitoneal shunt, a ventriculoatrial shunt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com