Conducting luminescent organic compounds
a luminescent organic compound and conductor technology, applied in the direction of non-metal conductors, cell components, conductors, etc., can solve the problems of inability to achieve sufficient molecular orientation, inability to use widely in practice, and decrease in the conduction path of charge carriers, etc., to achieve good solubility in solvents, impair the high field effect mobility, and facilitate the effect of mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples of form
[0194] A4 size or more.
Examples of Function:
[0195] Receiving and sending, data memory, switchover of picture plane, enlargement / reduction, input, search, image input, etc.
Examples of Constituent Element:
[0196] Display part, driving circuit, power source, communication (sending and receiving) circuit, control circuit, memory, input means, image input means, etc.
Examples of Application:
[0197]FIG. 17 shows an example of a display device for a global education system. The global education system as used herein includes an electronic blackboard 581 as the same display device as that of the electronic conference system of (7) and the same display device 582 as that of the electronic text of (19). By linking with schools around the world, the contents written on the electronic blackboard 581 by a teacher can be sent, displayed and recorded to the display device 582 of students. Individual communication between the teacher and a student can be supported. A voice input and output fu...
example 1
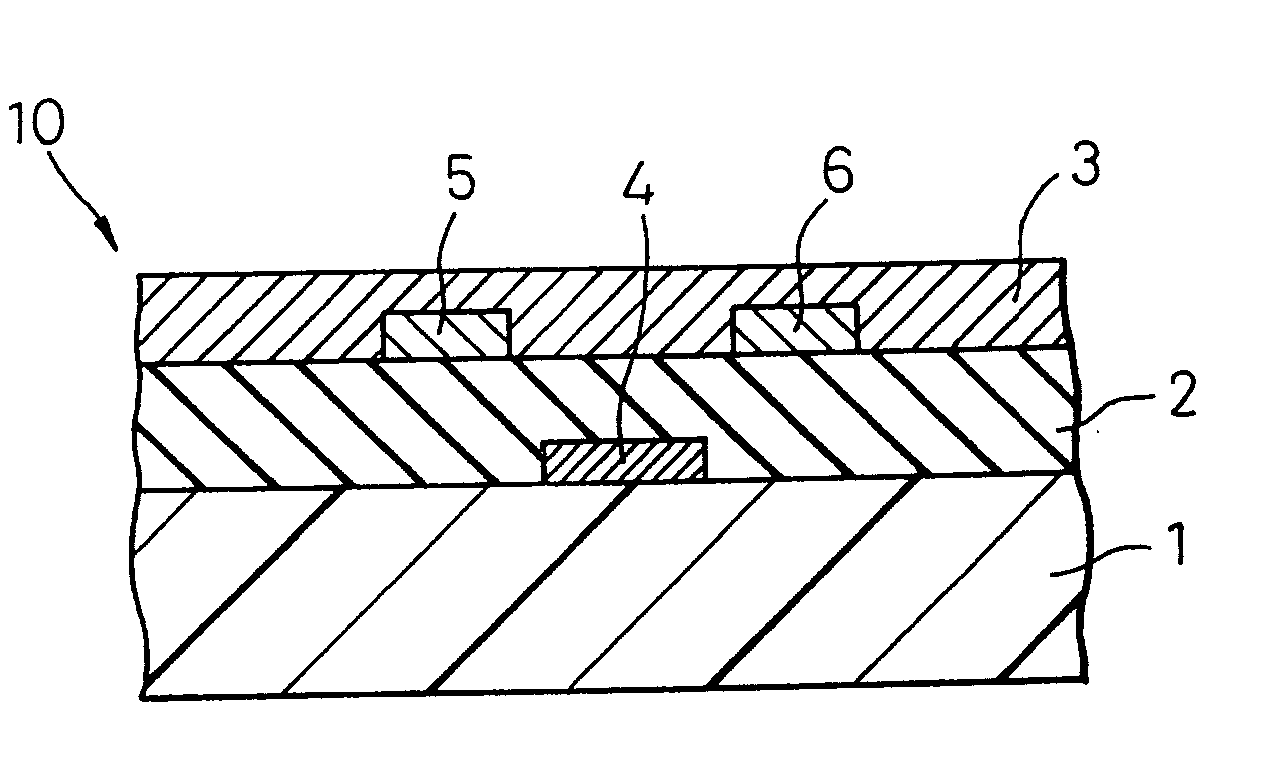
[0216] On a substrate plastic sheet comprising polyether sulfone, a gate electrode was formed by sputtering of gold, cyanoethylated pullulan dissolved in acetone was coated thereon, and then the solvent was dried to form an insulating layer having a film thickness of 150 nm. On this insulating layer, a source electrode and a drain electrode at an electrode-to-electrode distance, namely, a channel length, of 5 μm were each formed to a film thickness of 50 nm by sputtering of gold. On the plastic substrate with electrodes thus formed, an ovalene derivative having attached thereto a hexyl group (see, the following formula):
which was dissolved under heating in toluene was coated by spin coating and then, the solvent was gradually dried to obtain a channel layer having a film thickness of 100 nm.
[0217] Subsequently, for the thus-manufactured organic thin film transistor, the mobility of the electrically conducting organic compound was calculated from the relationships between the dra...
example 2
[0218] On a substrate plastic sheet comprising polyether sulfone, a gate electrode was formed by sputtering of gold and patterning, cyanoethylated pullulan dissolved in acetone was coated thereon, and then the solvent was dried to form an insulating layer having a film thickness of 150 nm. On this insulating layer, a source electrode and a drain electrode at an electrode-to-electrode distance, namely, a channel length, of 5 μm were formed each to a film thickness of 50 nm by sputtering of gold. On the plastic substrate with electrodes thus formed, a bianthrene derivative having attached thereto a hexyl group (see, the following formula):
which was dissolved under heating in toluene was coated by spin coating and then, the solvent was gradually dried while applying an electric field between the source-drain electrodes to obtain a channel layer having a film thickness of 100 nm.
[0219] Subsequently, for thus-manufactured organic thin film transistor, the mobility of the electrically...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com