Method and composition for diagnosis of melanocytic lesions
a technology of melanocytic lesions and compositions, applied in the field of melanocytic antigens and antibodies against melanomaassociated antigens, can solve the problems of limiting the usefulness of nki/c3 antibody in the diagnosis of melanocytic lesions, anti-s100 antibodies are notoriously unspecific, etc., and achieve the effect of increasing the solubility of compounds and facilitating the processing of active compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
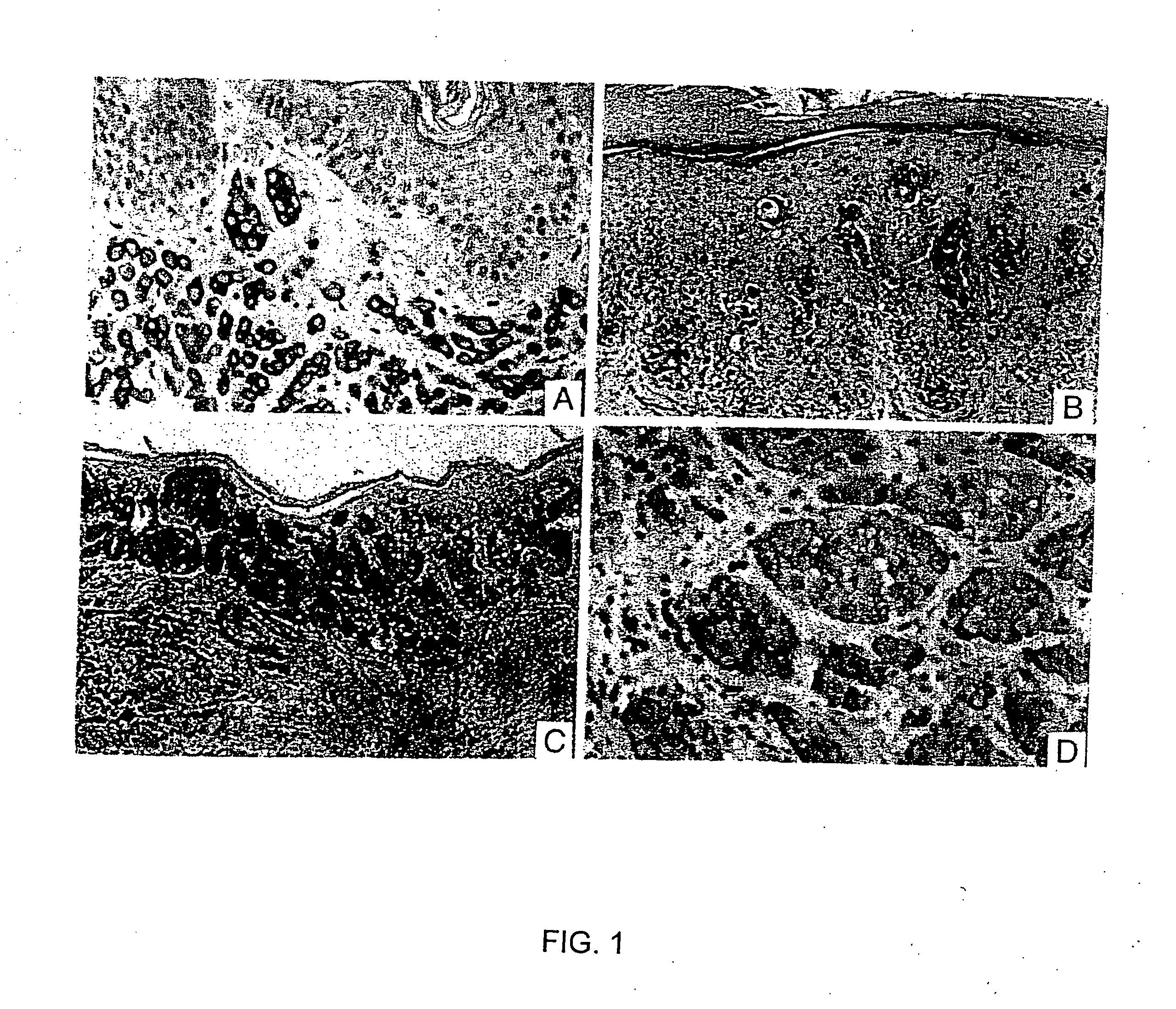
[0077] A. Preparation of SM5-1 Antibody
[0078] SM5-1 antibody is selected from a panel of monoclonal antibodies generated by using a subtractive imnunization protocol as previously described in Williams et al., (1992) Biotechniques 12:842-8477, and Brooks et al., (1993) J. Cell Biol. 22:1351-1359, both of which are incorporated by reference herein.
[0079] Briefly, mice were immunized with human melanoma cell line SMMU-1, which was obtained from the primary melanoma of a patient (Guo et al., (1994) Cancer Res. 54:561-1565, incorporated by reference herein). Next, the mice were treated with cyclophosphamide to abrogate activated B cells that produce antibody against epitopes expressed by the primary melanoma. The mice were then immunized with human melanoma cell line SMMU-2, which was obtained from the metastatic lesion of the same patient. The splenocytes from the mice were used for making hybridomas using standard techniques. Köhler G, Milstein C: Derivation of specific antibody pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| apparent molecular weight | aaaaa | aaaaa |
| spectrum | aaaaa | aaaaa |
| antibody titers | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com