Therapeutic agents for renal diseases and method for screening the same
a technology for applied in the field of renal diseases and therapeutic agents for renal diseases and the same screening method, can solve the problems of insufficient renal function, few therapeutic drugs effective for such purposes, and unacceptably strong adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of L-FABP Reporter Plasmid
[0053] (1) Isolation of Human FABP cDNA
[0054] Human L-FABP cDNA was isolated from human hepatocyte cDNA library (Clontech, Cat#HL1115b LOT#5621) by PCR (polymerase chain reaction) method. Primers were designed based on known sequence information (Lowe et. al., Journal of Biological Chemistry, vol. 260, pp. 3413-3417, 1985; Genbank / EMBL accession No. M10050) to result in fragment obtained by PCR having BamHI recognition sites at both ends. DNA fragment (about 420 base pairs) resulted from PCR contained the entire human L-FABP cDNA coding region. This fragment was ligated into an appropriate plasmid.
[0055] (2) Isolation of Human FABP Chromosomal Gene
[0056] Human L-FABP chromosomal gene was cloned from human chromosomal DNA library (Clontech, Cat#HL1006d LOT#5621) by plaque hybridization method using the fragment (about 420 base pairs, BamHI fragment) obtained in (1) above which includes human FABP cDNA as a probe. The resultant clone contained an ...
example 2
Primary Culture and Immortalization of Renal Tubular Cells, and Introduction of L-FABP Reporter Construct into the Same
[0062] (1) Isolation of Nephron
[0063] A nephron was isolated from mouse kidney by microdissection method in the following manner. A mouse was anesthetized with pentobarbital before laparotomy and perfused with 20 ml of cold Hank's buffered solution (HBS) containing 0.1% BSA via abdominal artery. Then, 10 ml of cold HBS containing 0.1% BSA and 0.1% collagenase type 1 was perfused. These perfusion solutions were previously saturated with a gas mixture of 5% CO.sub.2-95% O.sub.2. Kidneys were then isolated and cut into slice sections of 0.5-1.0 mm thick with Surgical Brade. These sections were soaked into 10 ml of 0.1% collagenase in HBS, incubated at 37.degree. C. for 10 minutes for enzymatic treatment and then washed with HBS twice. A single segment of nephrons was isolated from these slice sections while observing with stereomicroscope under ice cooling.
[0064] (2) P...
example 3
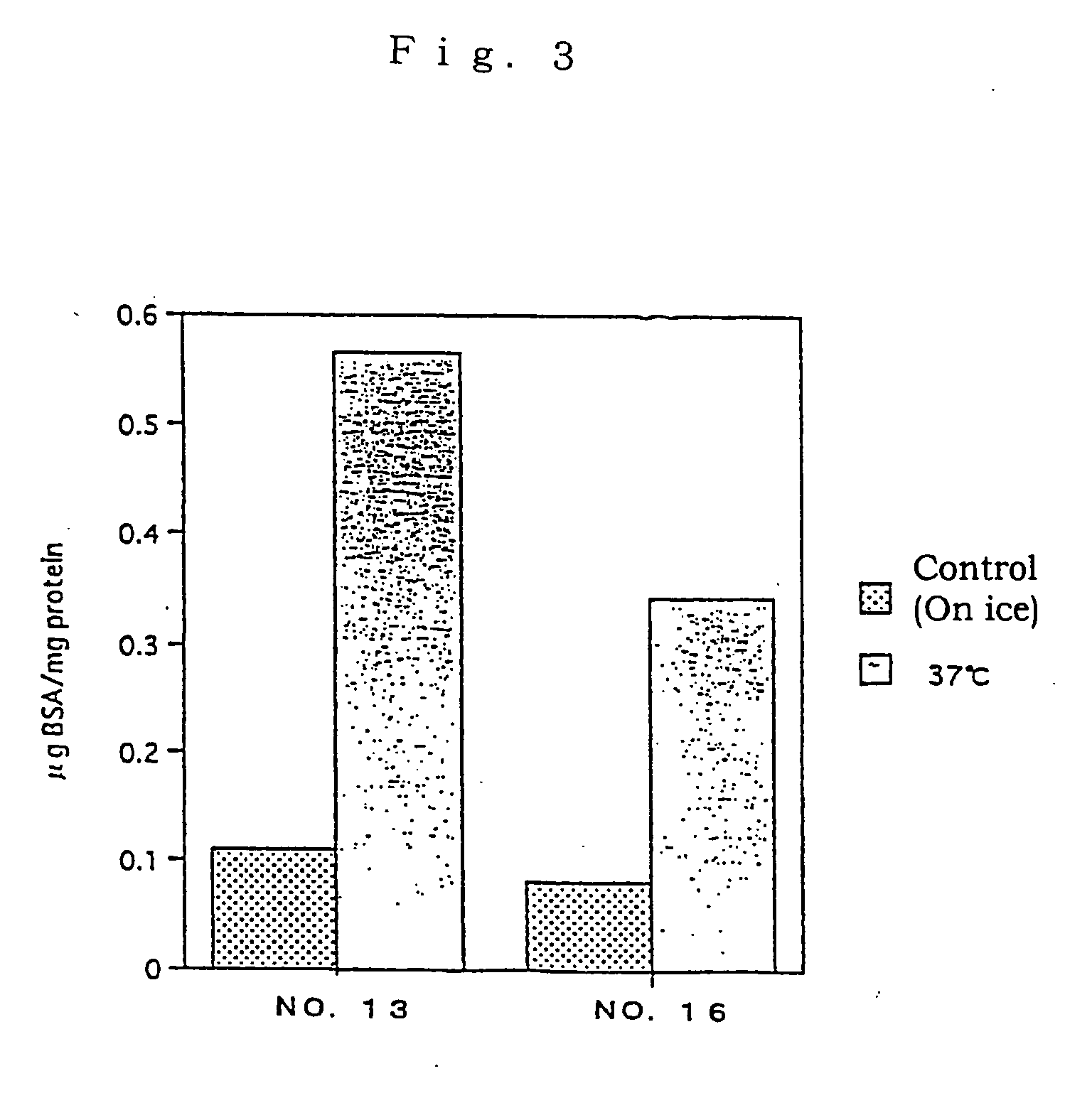
Assay of L-FABP Up-Regulating Activity in Proximal Renal Tubular Epithelial Cells
[0086] A test substance was examined for the L-FABP up-regulating activity by reporter assay method in the following manner using the proximal renal tubular epithelial cell line mProx 37-13 (also referred as "cell line No.13") comprising L-FABP reporter DNA construct and was prepared in Example 2 above.
[0087] Firstly, the cell line mProx 37-13 was added in 96-well flat-bottom plates at 5.times.10.sup.4 cells / 100 .mu.l / well and cultured. The cultivation was conducted using plates pre-coated with 0.1% gelatin and, as a medium, a high glucose-containing Dulbecco's MEM medium (Dulbecco's MEM, high glucose; DMEM) (Gibco) supplemented with 10% fetal calf serum, 100 unit / ml of penicillin and 100 .mu.g / ml of streptomycin. After 48-hour-cultivation, the plate was washed once with serum-free medium, and a serum-free medium containing test substance was added thereto. For control samples, no test substance was add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com