Method for treatment of demyelinating central nervous system disease
a central nervous system and demyelinating technology, applied in the direction of immunological disorders, drug compositions, peptide/protein ingredients, etc., can solve the problems of progressive neurological impairment, short circuit of electrical pathways, loss of electrical insulation, etc., to prevent demyelinating central nervous system diseases, treat and/or diminish demyelinating central nervous system symptoms, and reduce the symptoms of multiple sclerosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
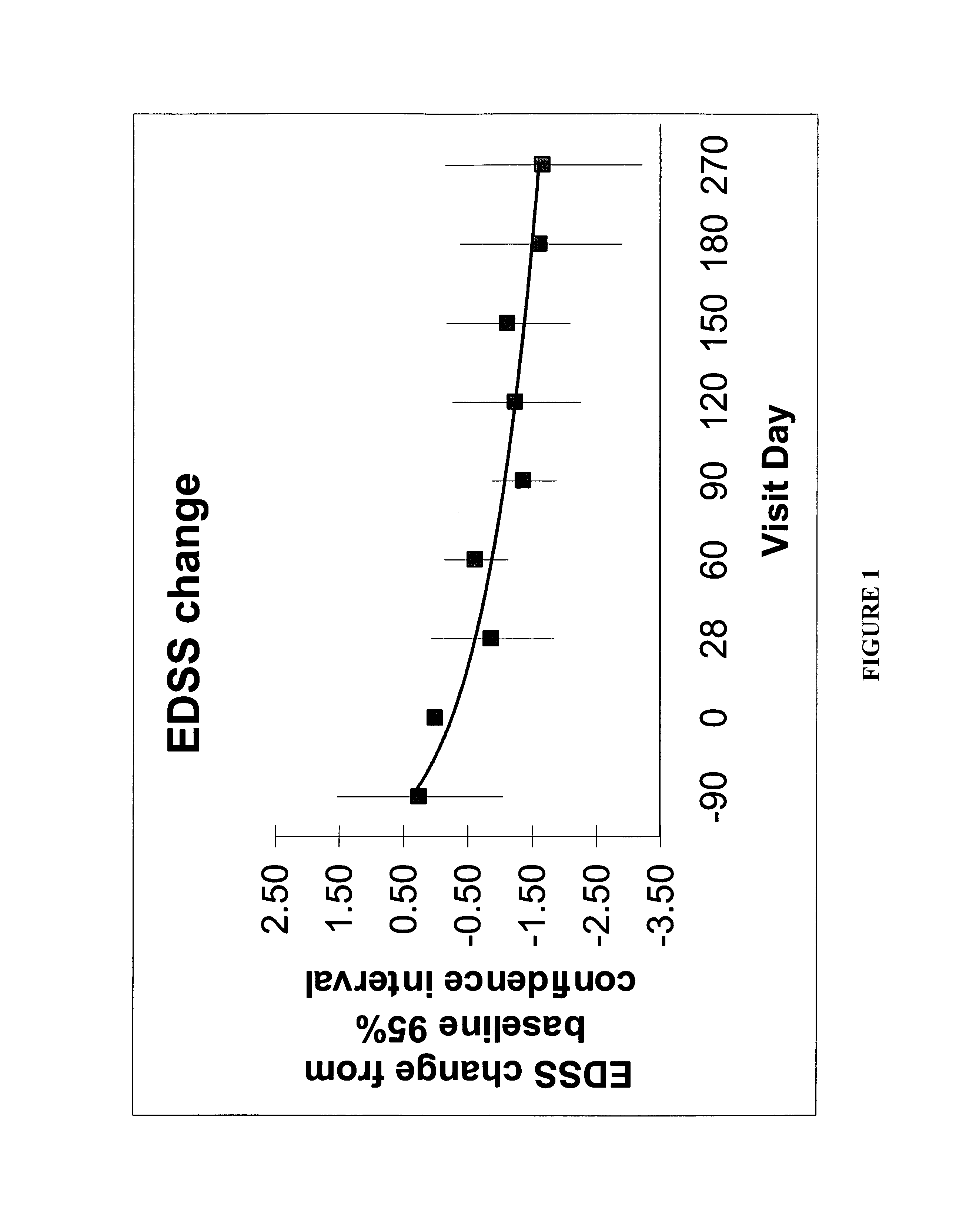
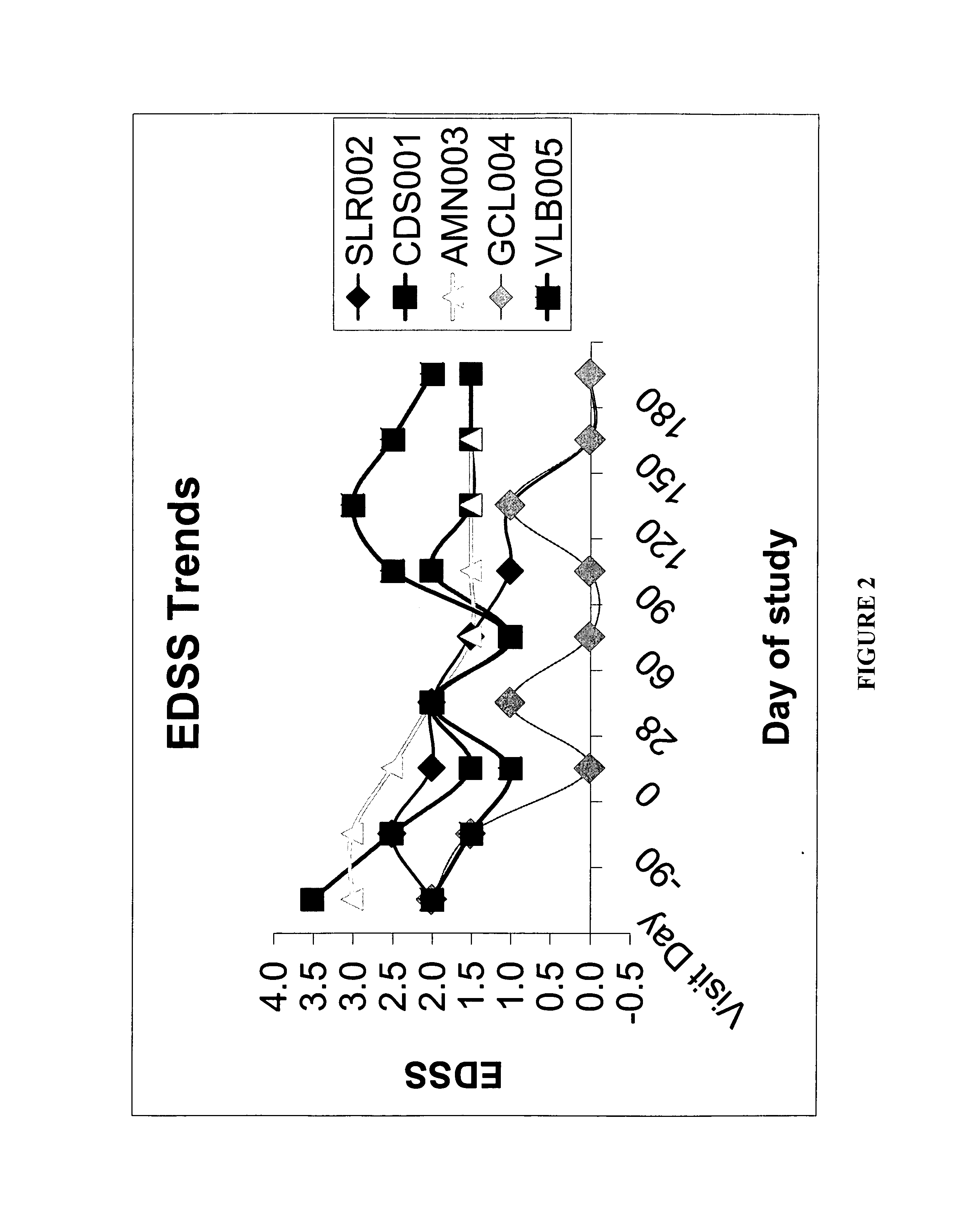
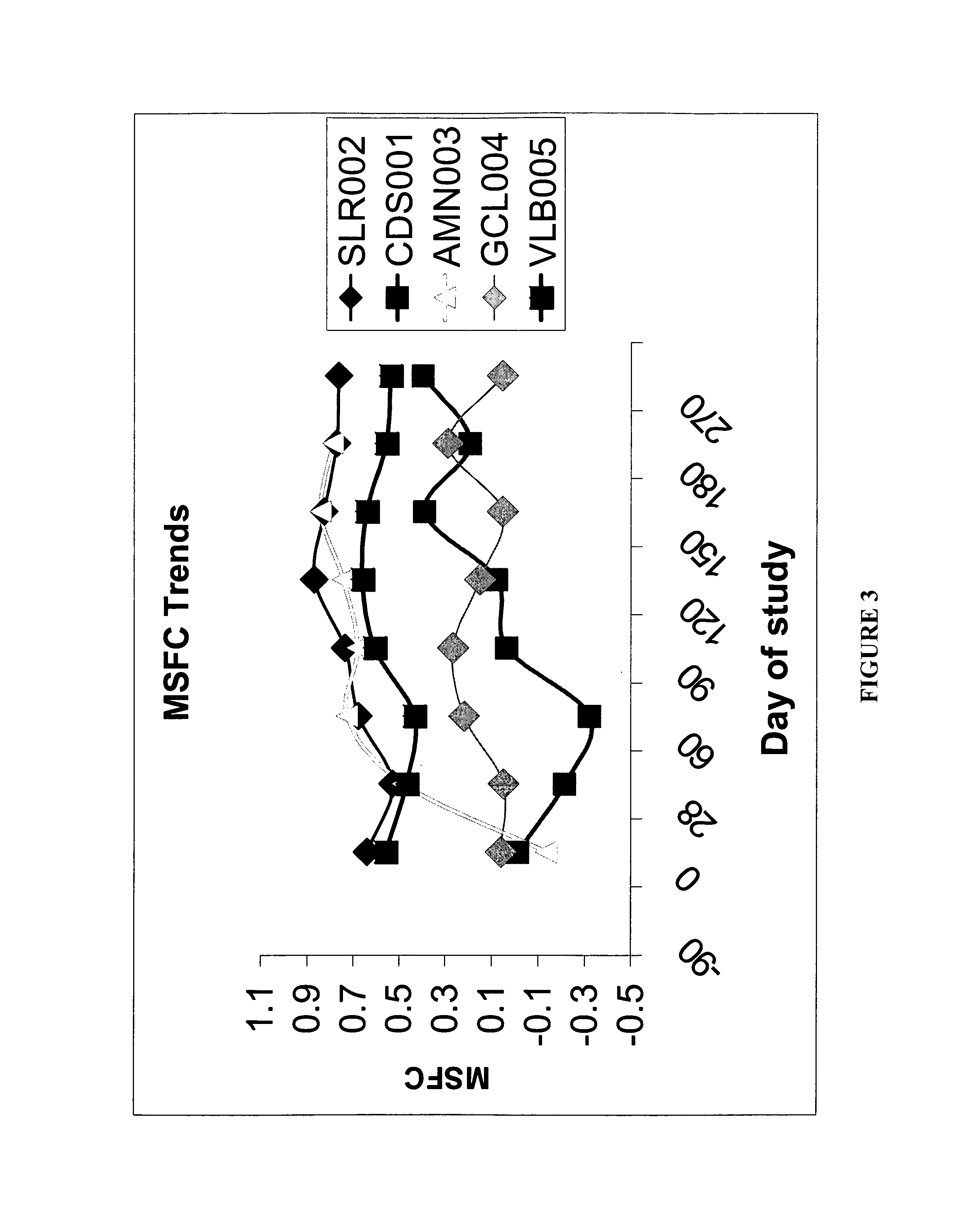
[0043] This Example helps demonstrate the effectiveness of the methods of the present invention. In the embodiment discussed in this Example, CSF was used in combination with an interferon-beta-1a treatment. The drawing sheets demonstrate the level of disability for individuals with relapsing-remitting MS during treatment with sargramostim at doses of either about 250 mcg or the maximally tolerated dose subcutaneously three times a week. Each individual was simultaneously being treated with a weekly interferon-beta-1a 30 micrograms intramuscularly. Both the Kurtzke Extended Disability Status Score (EDSS) and the MS Functional composite (MSFC) score were employed for monitoring disability. See FIGS. 1 and 2. The subjects were individuals with mild disability from multiple sclerosis who were already treated for 90 days with an FDA-approved immunomodulatory therapy at time of initiation of CSF treatment, ninety days following initiation of interferon. This dose of interferon is general...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com