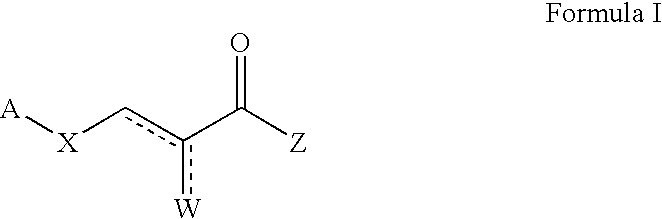
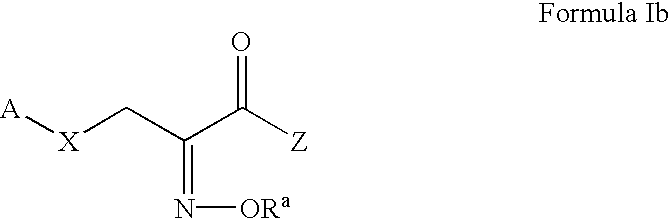
Pyruvate derivatives
a technology of pyruvate and derivatives, applied in the field of pyruvate derivatives, can solve the problems of not being able to distinguish between "preventing" and "suppressing" and being sterically impractical and/or synthetically non-feasibl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
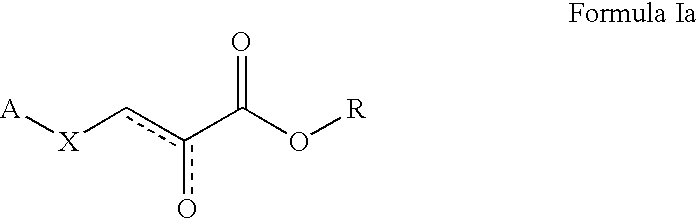
Formula Ia where A is 1H-Benzimidazole-2-yl, R is Ethyl, and X is S
[0592] A solution of 2-mercaptobenzimidazole (200 mg, 1.33 mmol) and ethyl 3-bromopyruvate (0.20 mL, 1.43 mmol) in methanol (2.5 mL) and acetone (2 mL) was shaken at 20.degree. C. for 4 hours. The solvents were removed under the reduced pressure on a rotary evaporator. The residue was triturated with ethyl acetate. Solvent was decanted and the residue was dissolved in methylene chloride. The solution was washed with diluted aqueous sodium bicarbonate solution and water, and then dried over magnesium sulfate. Removal of the solvent gave the expected product, 3-(1H-Benzoimidazol-2-ylsulfanyl)-2-oxo-propionic acid ethyl ester (326 mg, 93%). .sup.1H-NMR (CDCl.sub.3, 300 MHz) .delta. (ppm): 7.31 (br. s, 1H), 7.24 (br. s, 1H), 7.15-7.05 (m, 2H), 4.32 (q, J=7.1 Hz, 2H), 4.29 (br. s, 1H), 3.90 (br. s, 1H), 1.24 (t, J=7.1 Hz, 3H). MS (ESI): m / z 265 (M+1, 100).
example 2
Formula Ia where A is 5-Methoxy-1H-benzimidazole-2-yl, R is Ethyl, and X is S
[0593] A solution of 2-mercapto-5-methylbenzimidazole (200 mg, 1.22 mmol) and ethyl 3-bromopyruvate (0.20 mL, 1.43 mmol) in methanol (2 mL) and acetone (3 mL) was shaken at room temperature for 4 hours. The solvents were removed under the reduced pressure on a rotary evaporator. The residue was triturated with ethyl acetate. Solvent was decanted and the residue was dissolved in methylene chloride. The solution was washed with diluted aqueous sodium bicarbonate solution and water, and then dried over magnesium sulfate. Removal of the solvent gave the expected product, 3-(5-methoxy-1H-benzoimidazol-2-ylsulfanyl)-2-oxo-propionic acid ethyl ester (300 mg, 88%). .sup.1H-NMR (D.sub.3COD, 300 MHz) .delta. (ppm): 7.19 (br. s, 1H), 7.10 and 7.04 (br. 2 s, 1H), 6.94 (d, J=8.2 Hz, 1H), 4.34 (q, J=7.1 Hz, 2H), 4.31 (br. s, 1H), 3.91 (br. s, 1H), 2.37 (br. s, 3H), 1.26 (t, J=7.1 Hz, 3H). MS (ESI): m / z 279 (M+1, 100).
example 3
Formula Ia where A is 5-Methyl-1H-benzimidazole-2-yl, R is Ethyl, and X is S
[0594] A solution of 5-methoxy-2-benzimidazolethiol (200 mg, 1.11 mmol) and ethyl 3-bromopyruvate (0.20 mL, 1.43 mmol) in methanol (5 mL) and acetone (2 mL) was shaken at room temperature for 4 hours. The solvents were removed under the reduced pressure on a rotary evaporator. The residue was triturated with ethyl acetate. Solvent was decanted and the residue was dissolved in methylene chloride. The solution was washed with diluted aqueous sodium bicarbonate solution and water, and then dried over magnesium sulfate. Removal of the solvent gave the expected product, 3-(5-Methyl-1H-benzoimidazol-2-ylsulfanyl)-2-oxo-propionic acid ethyl ester (300 mg, 92%). .sup.1H-NMR (D.sub.3COD, 300 MHz) .delta. (ppm): 7.19 (br. s, 1H), 6.75 (br. s, 1H), 6.69 (dd, J=2.3 Hz, J=8.8 Hz, 1H), 4.30 (q, J=7.1 Hz, 2H), 4.29 (br. s, 1H), 3.93 (br. s, 1H), 3.69 (s, 3H), 1.24 (t, J=7.1 Hz, 3H). MS (ESI): m / z 295 (M+1, 100).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com