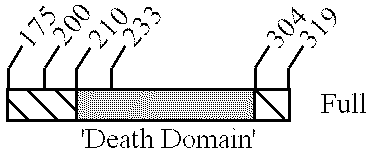
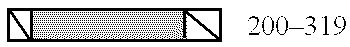
Modulators of the function of FAS/APO1 receptors
a technology of fas/apo1 and receptors, which is applied in the field of modulators of the function of fas/apo1 receptors, can solve the problems of little understanding of the mechanism of triggering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
: Cloning and isolation of the MORT-1 protein which binds to the intracellular domain of the FAS-R
(i Two-hybrid screen and two-hybrid .beta.-galactosidase expression test
[0113] To isolate proteins interacting with the intracellular domain of the FAS-R, the yeast two-hybrid system was used (Fields and Song, 1989; see also co-pending IL 109632, 112002 and 112692). Briefly, this two-hybrid system is a yeast-based genetic assay to detect specific protein-protein interactions in vivo by restoration of a eukaryotic transcriptional activator such as GAL4 that has two separate domains, a DNA binding and an activation domain, which domains when expressed and bound together to form a restored GAL4 protein, is capable of binding to an upstream activating sequence which in turn activates a promoter that controls the expression of a reporter gene, such as lacZ or HIS3, the expression of which is readily observed in the cultured cells. In this system the genes for the candidate interacting protei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com