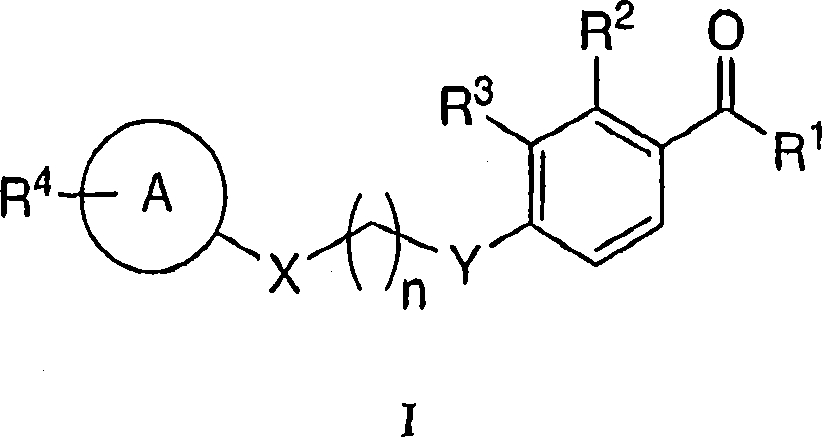
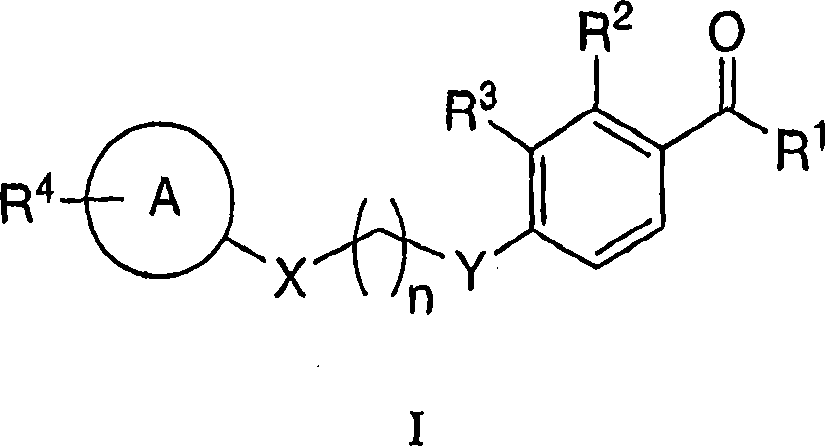
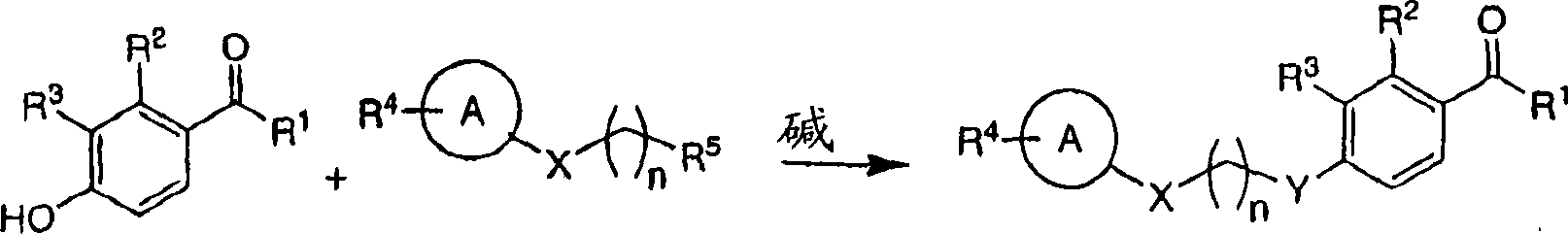
Heterocyclic acetophenone potentiators of metabotropic glutamate receptors
An azetidinyl and phenyl technology, applied in the field of mGluR2 receptor synergists, can solve problems such as phosphoinositide hydrolysis and increased intracellular calcium transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220]
[0221] 7-{4-[3-Hydroxy-2-methyl-4-(3-methyl-butyryl)-phenoxy]-butoxy}-chromen-2-one
[0222] Under stirring at 45°C, potassium carbonate (2.39g, 17.3mmol) was added to 1-(2,4-dihydroxy-3-methyl-phenyl)-3-methyl-butan-1-one (150mg , 0.68mmol) and 1,4-dibromobutane (6.22g, 28.8mmol) in acetone (100mL). The reaction mixture was stirred for 16 h, then the acetone was removed in vacuo. The residue was then mixed with dichloromethane (100 mL) and water (100 mL). The organic layer was separated and washed with MgSO 4 Drying and concentration in vacuo gave a residue which was purified by column chromatography on silica gel (eluting with 0-60% ethyl acetate / hexanes) to afford 3.26 g (98%) of 1-[4-(4-bromo-butan oxy)-2-hydroxy-3-methyl-phenyl]-3-methyl-butan-1-one as a white solid. Potassium carbonate (161 mg, 1.16 mmol) was then added to 1-[4-(4-bromo-butoxy)-2-hydroxy-3-methyl-phenyl]-3-methanol with stirring at 45°C Butan-1-one (200 mg, 0.58 mmol) and 7-hydroxycoumar...
Embodiment 2
[0225]
[0226] 1-[2-Hydroxy-3-methyl-4-(4-phenoxy-butoxy)-phenyl]-3-methyl-butan-1-one
[0227] Under stirring at 45°C, potassium carbonate (398 mg, 12.88 mmol) was added to 1-(2,4-dihydroxy-3-methyl-phenyl)-3-methyl-butan-1-one (300 mg, 1.44mmol) and (4-bromo-butoxy)-benzene (396mg, 1.73mmol) in acetone (20mL). The reaction mixture was stirred for 16 h, then the acetone was removed in vacuo. The residue was then mixed with dichloromethane (50 mL) and water (50 mL). The organic layer was separated and washed with MgSO 4 Drying and concentration in vacuo gave a residue which was purified by column chromatography on silica gel (eluting with 0-60% ethyl acetate / hexanes) to give 381 mg (74%) of 1-[2-hydroxy-3-methyl- 4-(4-Phenoxy-butoxy)-phenyl]-3-methyl-butan-1-one as a colorless oil.
[0228] 1 H NMR (CDCl 3 , 500MHz), δ13.04(s, 1H), 7.62(d, 1H), 7.32-7.28(m, 2H), 6.98-6.91(m, 3H), 6.45(d, 1H), 4.16-4.07(m , 4H), 2.79(d, 2H), 2.30-2.28(m, 1H), 2.10(s, 3H), 2.07-2.03(m...
Embodiment 3
[0230]
[0231] 1-[3-Bromo-2-hydroxy-4-(4-phenoxy-butoxy)-phenyl]-3-methyl-butan-1-one
[0232] A procedure similar to that outlined in Example 2 was followed, starting with 1-(3-bromo-2,4-dihydroxy-phenyl)-3-methyl-butan-1-one.
[0233] 1 H NMR (CDCl 3 , 500MHz), δ13.57(s, 1H), 7.23(d, 1H), 7.31-7.28(m, 2H), 6.97-6.91(m, 3H), 6.50(d, 1H), 4.22(t, 2H ), 4.09(t, 2H), 2.80(d, 2H), 2.31-2.28(m, 1H), 2.11-2.05(m, 4H), 1.02(d, 6H). MS(ESI): 421(M+ h)+ .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com