Multiple-variable dose regimen for treating tnfalpha-related disorders
A dose and symptom technology, applied in the field of variable doses in the treatment of Crohn's disease, can solve the problem of inability to completely reduce inflammatory side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0478] Example 1: Efficacy Study of Multiple Variable Dose Therapy for the Treatment of Crohn's Disease
[0479] Multiple-dose treatment approach for Crohn's disease using D2E7
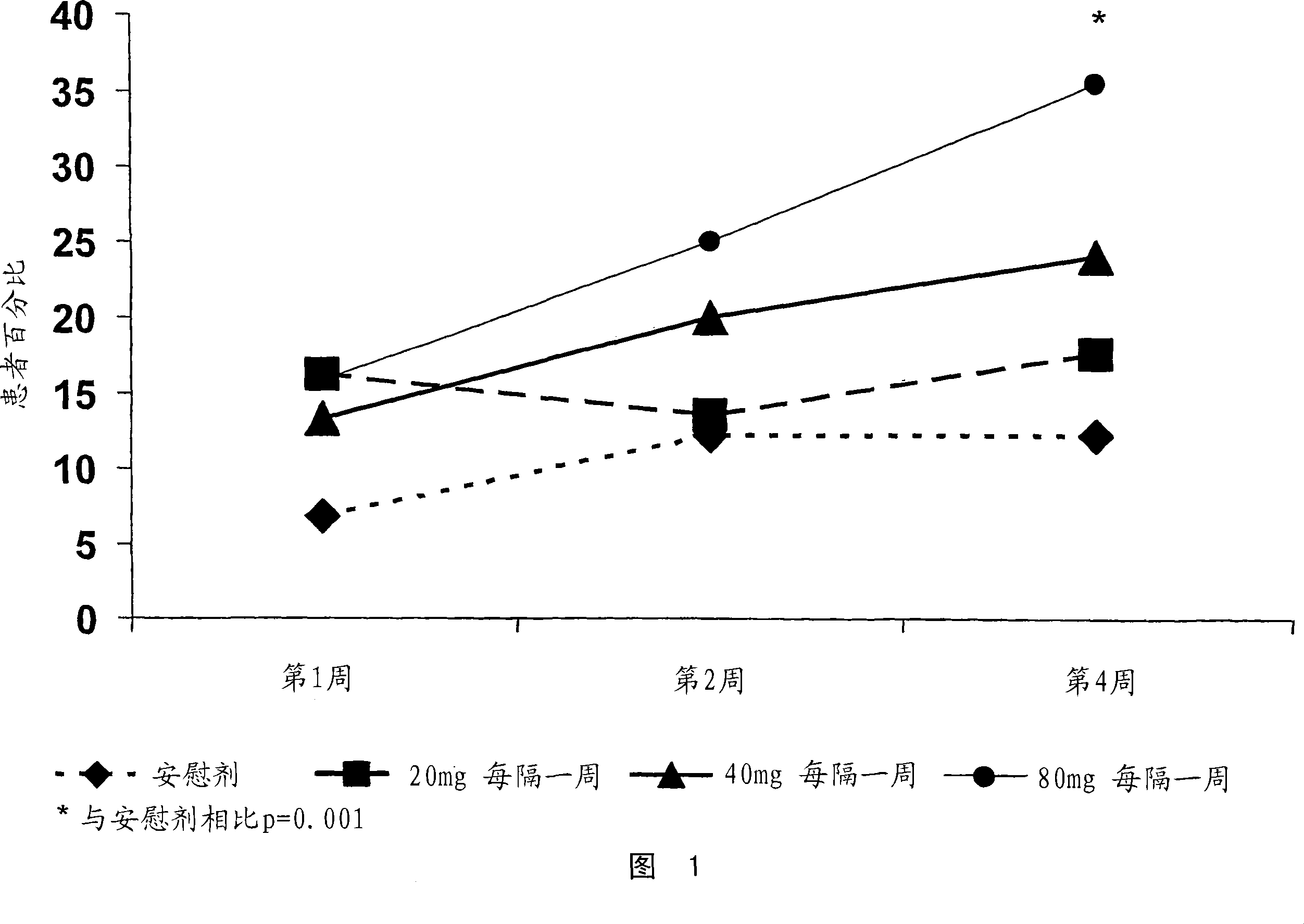
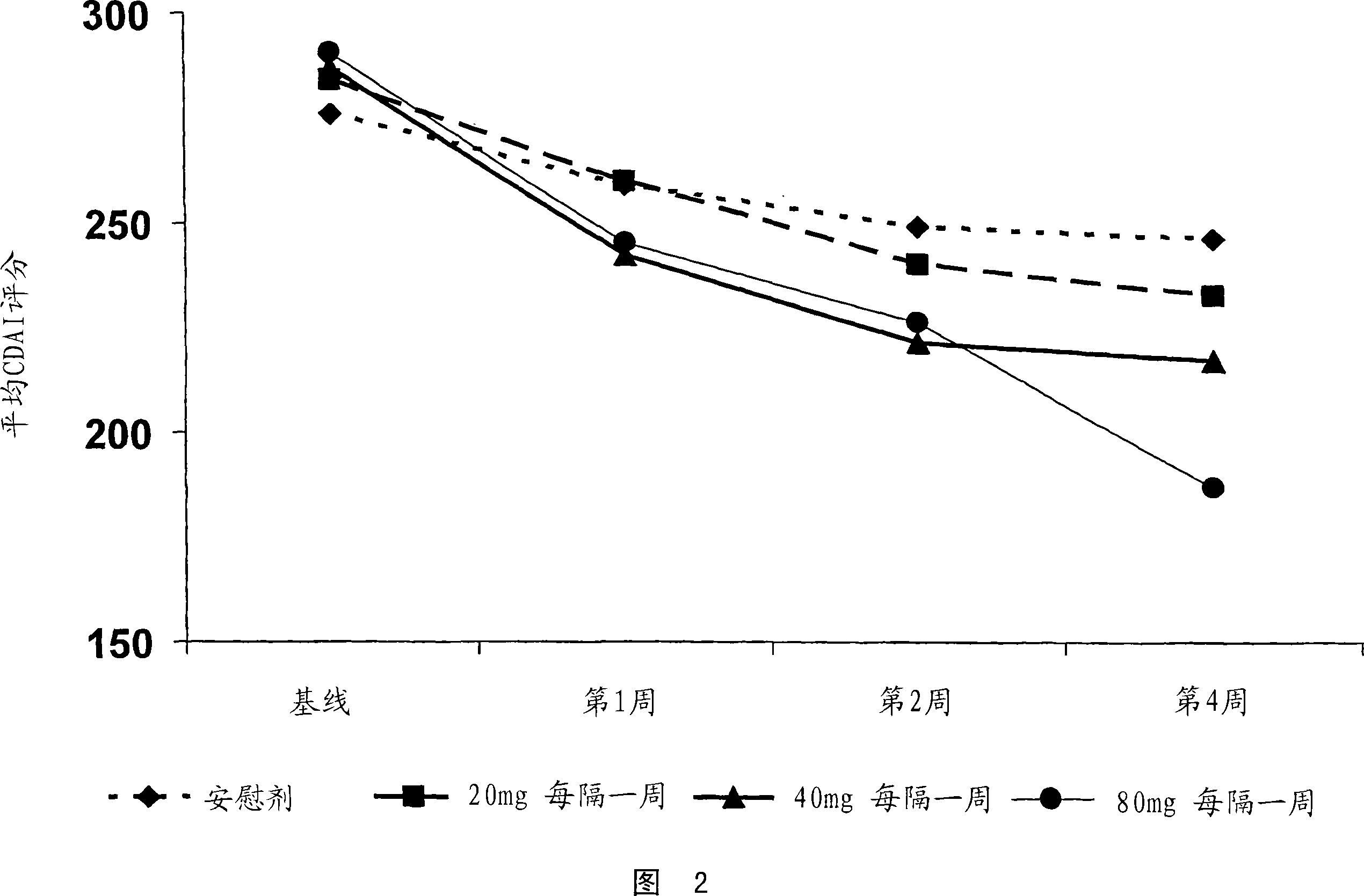
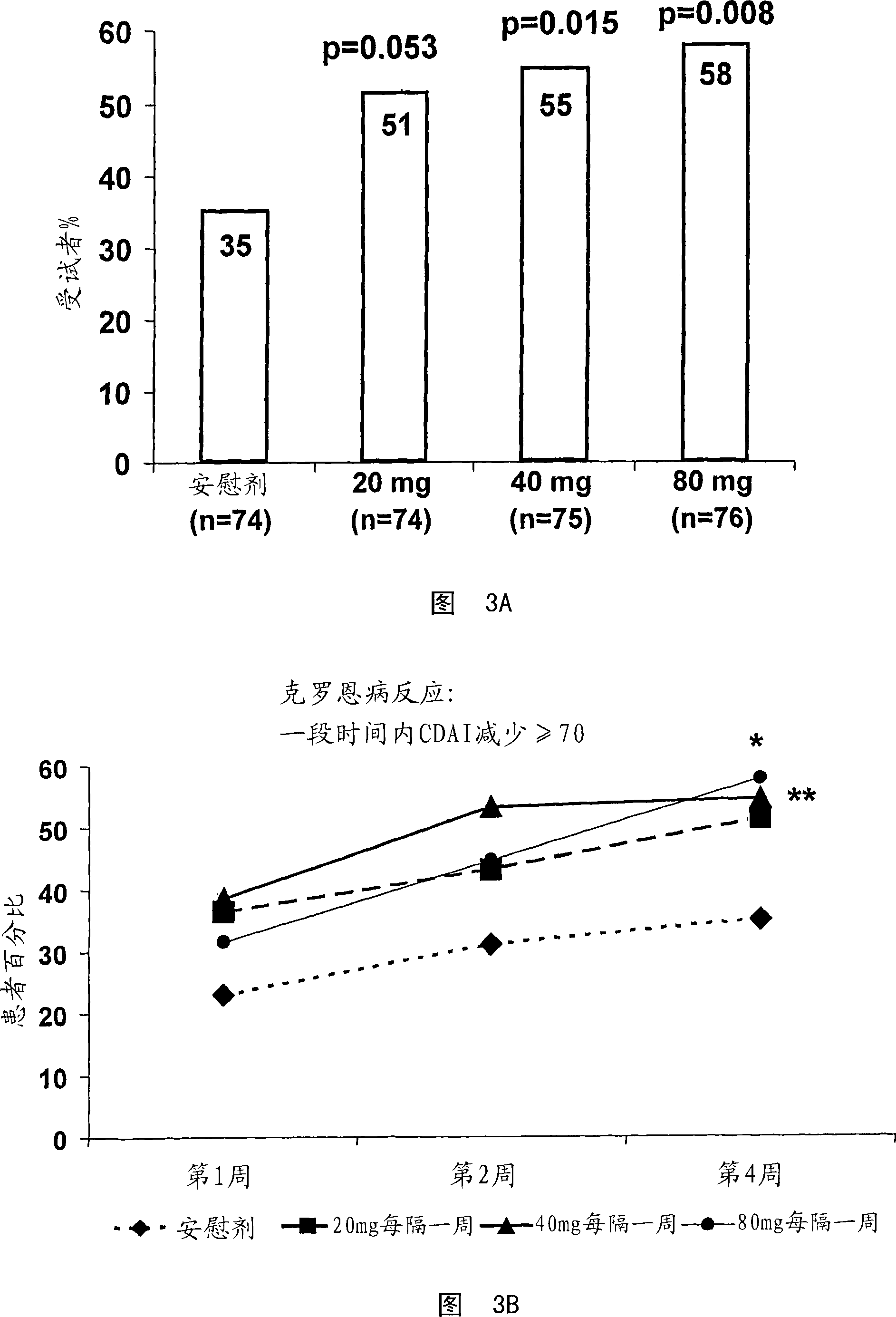
[0480] A study was conducted to determine the TNFα inhibitor D2E7 (also known as Adalimumab and Humira ) in the treatment of Crohn's disease. The efficacy and tolerability of D2E7 in the treatment of patients with acute Crohn's disease were evaluated in a randomized double-blind placebo-controlled multicenter study.
[0481] In this study, 299 TNF-antagonist naïve patients with active Crohn's disease were selected. Crohn's disease was confirmed in each patient by endoscopic or radiological evaluation. Subjects were randomly and equally assigned to one of 4 treatment groups (3 treatments and 1 placebo group). Eligible subjects included men and women aged 18-75 with a Crohn's disease diagnosis for more than 4 months. In addition, selected patients had active Crohn's disease, defined as a...
Embodiment 2
[0495] Example 2: Additional studies of the efficacy of multiple variable dose therapy for the treatment of Crohn's disease
[0496] Multiple-dose treatment approach for Crohn's disease using D2E7
[0497] A study was conducted to evaluate the tolerability and clinical benefit of multiple variable dose treatment with TNFα inhibitors, particularly D2E7, in adult patients with Crohn's disease who had previously received and responded to different TNFα inhibitors. Patients who had previously received the chimeric anti-TNF antibody infliximab but no longer had a sustained response and / or tolerance to infliximab were included in the study.
[0498] Patients who had become unresponsive or developed intolerance (acute or delayed infusion reactions) were treated with D2E7 80 mg at week 0 and 40 mg at week 2. All treatments were performed subcutaneously. Antibody to Infliximab (ATI) at baseline was determined (Prometheus Laboratories, San Diego, CA). Crohn's disease activity...
Embodiment 3
[0501] Example 3: Efficacy of multiple variable dose therapy with TNFα inhibitors in the treatment of psoriasis
[0502] Multiple-dose treatment approach for psoriasis using D2E7
[0503] A study was conducted to determine the efficacy of a multiple variable dosage regimen of D2E7 in the treatment of psoriasis. The efficacy and tolerability of D2E7 in the treatment of patients with moderate to severe chronic plaque psoriasis were evaluated in a randomized double-blind placebo-controlled multicenter study.
[0504] In this study, 148 adult patients diagnosed with moderate-to-severe psoriasis for at least 1 year were selected to receive multiple-variable dose therapy. Patients were also selected on the basis of > 5% body surface area (BSA) affected. Subjects were randomly and equally assigned to one of three groups (two treatment groups and one placebo group).
[0505] At baseline (week 0), patients in both treatment groups received an induction dose of 80 mg D2E7. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com