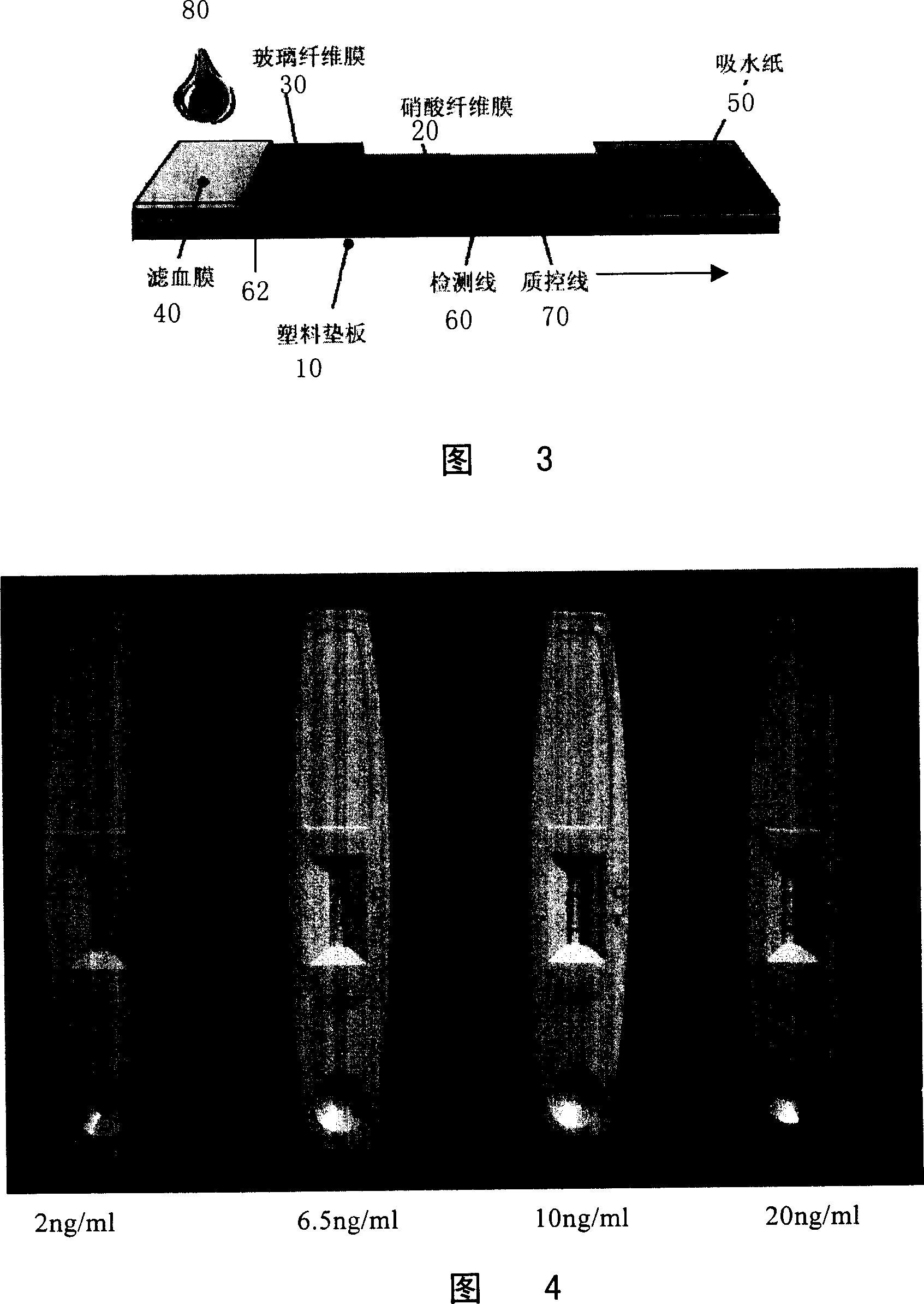
Method for preparing heart fatty acid binding protein monoclonal antibody and its use
A combination technology of monoclonal antibody and fatty acid, applied in the direction of anti-animal/human immunoglobulin, measuring devices, instruments, etc., to achieve high-precision results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Preparation of monoclonal antibodies by hybridoma cell method:
[0083] A. Antibody Preparation
[0084] 1. The BalB / C mice were immunized by a conventional method, and the immunogen was natural H-FABP protein isolated and purified from human heart tissue (purchased from Life Diagnostic Company of the United States);
[0085] 2. For the first immunization, fully emulsify 100 micrograms of the antigen with Freund's complete adjuvant, and then inject it intraperitoneally or subcutaneously at multiple points. After that, every two weeks, inject 50 micrograms of antigen mixed with incomplete adjuvant, and immunize four times in total;
[0086] 3. Immunization was boosted once 3 days before fusion, and then the spleen was taken out to prepare a spleen cell suspension;
[0087] 4. fused with SP2 / 0 cells under the action of PEG fusion agent, and screened single clones on HAT selection medium;
[0088] 5. After several fusions, 15 anti-H-FABP specific monoclonal antibody str...
Embodiment 2
[0106] Preparation of monoclonal antibody by mouse ascites method:
[0107] A. Antibody preparation:
[0108] 1. The mice used to prepare ascites are all SPF grade mice. The production mice have been inspected and have qualified certificates. If the animals are unhealthy, bitten or infected during the production process, they will be discarded immediately.
[0109] 2. Animal equipment and facilities: All animal experiments were carried out in the Animal Center of Shanghai Second Medical University, which is an SPF animal room and complies with national regulations.
[0110] 3. Establishment of cell bank: In order to ensure the long-term and stable production of high-quality monoclonal antibodies that meet the requirements, a high-standard cell bank must first be established, including the original cell bank, seed cell bank and production cell bank, the location and generation of cell tubes Special personnel are responsible for the number of tubes, cryopreservation, and resusc...
Embodiment 3
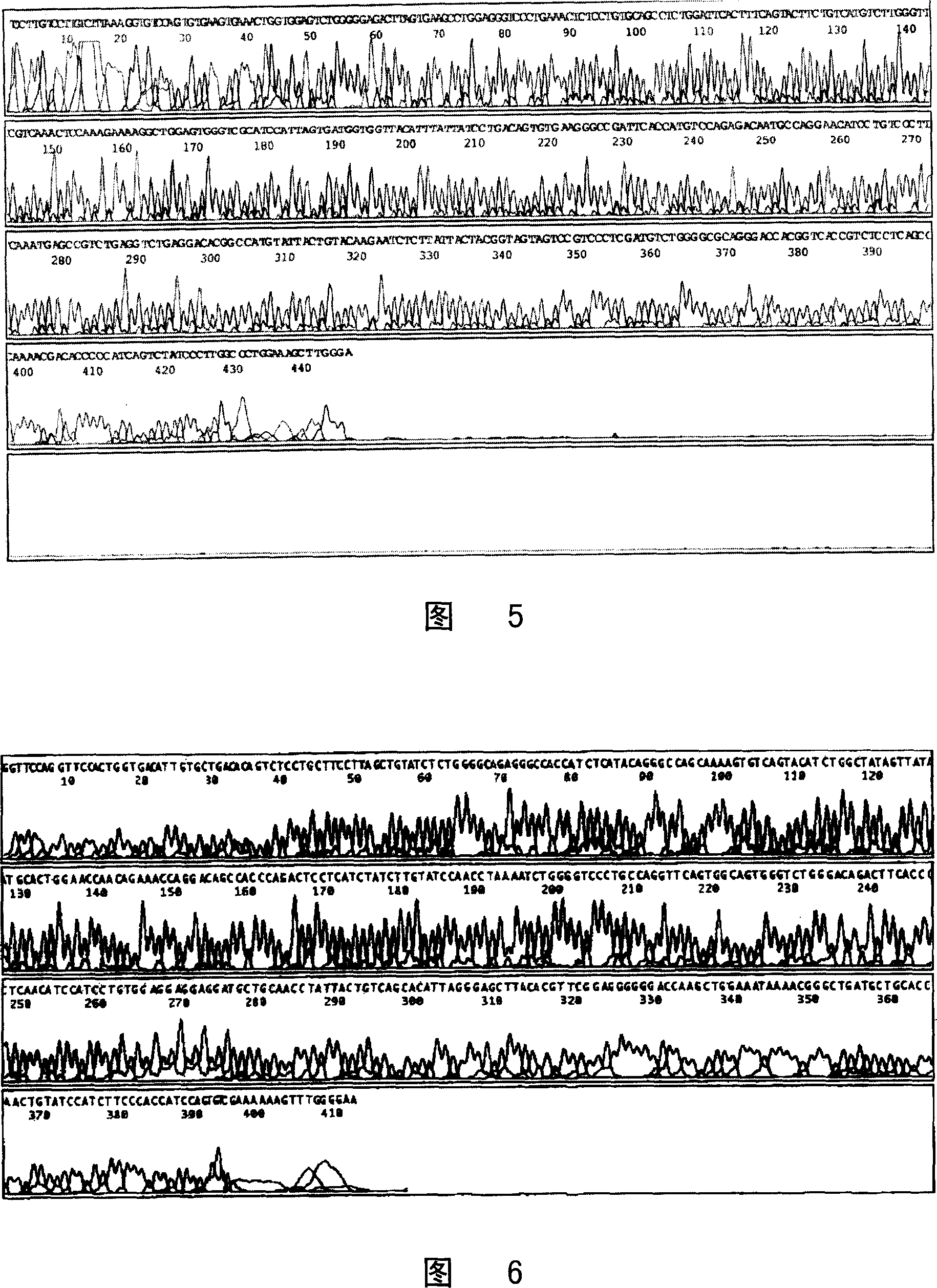
[0121] Antibody Sequencing
[0122] The currently applied rapid sequencing technique is the enzymatic method proposed by Sanger et al. (1977). Firstly, several groups of fluorescently labeled oligonucleotides are synthesized independently of each other. Each group of oligonucleotides has a fixed starting point, but ends randomly at one or more specific residues. Since each base on the DNA has an equal chance of appearing at the variable termination end, each of the above-mentioned products is a mixture of oligonucleotides whose length is determined by a specific base in the original DNA. Determined by the position on the fragment. Then, under conditions that can distinguish different DNA molecules with a length difference of only one nucleotide, electrophoresis analysis is performed on each group of oligonucleotides, as long as several groups of oligonucleotides are added to several adjacent DNA molecules in the sequencing gel. On the swimming lane, the nucleotide sequence o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com