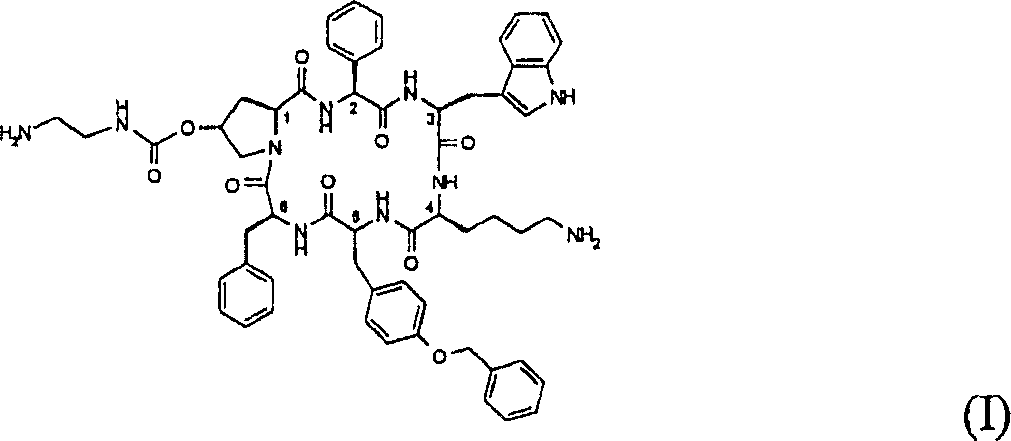
Biomarkers for the efficacy of somatostatin analogue treatment
A growth-stimulating hormone and biomarker technology, applied in the field of gene expression profiling for growth regulation, can solve the problems of insufficient growth control and contraindication of surgical operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0038] Gene expression profiles in pasireotide-induced monkeys
[0039] Foreword and Summary
[0040] Microchip gene expression analysis was performed using monkey tissues treated with subtherapeutic doses of pasireotide for 14 days. The results of these assays were analyzed to identify the mode of action of pasireotide relevant for therapeutic applications.
[0041] All monkey tissues examined (thyroid, brown fat, pituitary, pancreas, liver, kidney, spleen) demonstrated the association of natural somatostatin 14 (SST-14) and somatostatin 28 (SST-28) with growth-promoting Changes in genes regulated by statin receptor (SSTR) binding. The transcriptional profile reflects the known effects of somatostatin on growth hormone / insulin-like growth factor 1 (GH / IGF-1), the glucagon / insulin axis, and on cell proliferation. However, the compounds significantly affected the transcriptional levels of other related genes such as insulin-like growth factor 2 (IGF-2) in the pituitary and k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com