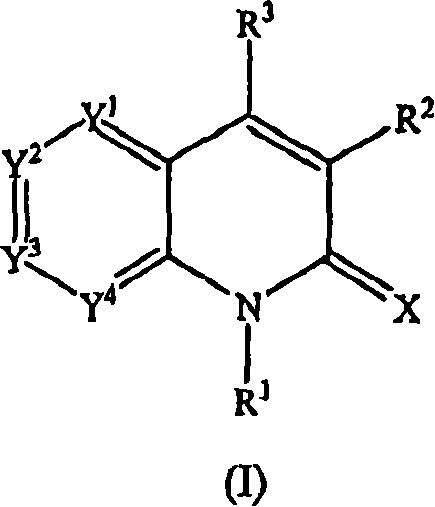
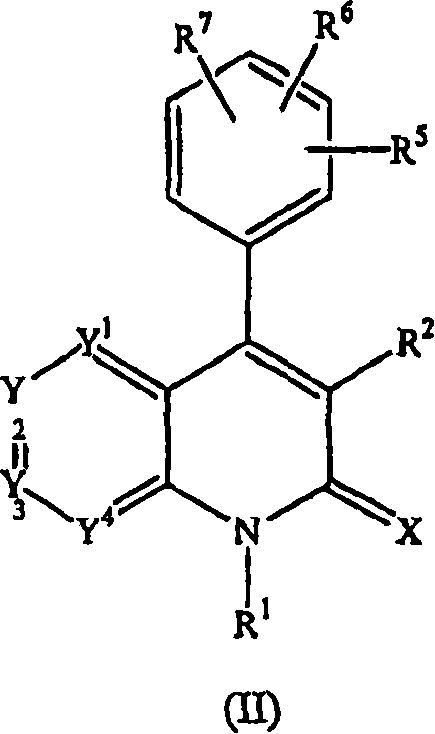
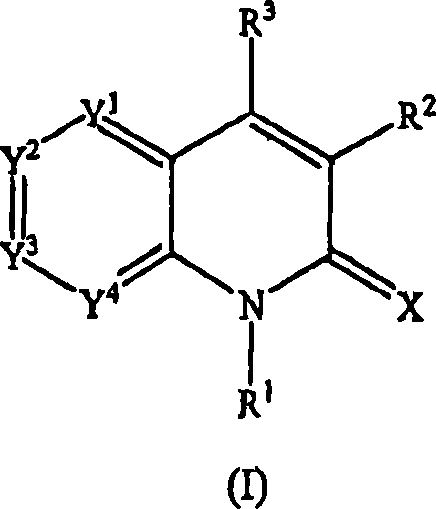
Quinolinone derivatives as inhibitors of c-fms kinase
A solvate and compound technology, applied in the field of quinolinone derivatives as c-fms kinase inhibitors, can solve problems such as oncogene transcription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] Example 1: General method for the synthesis of quinolones and naphthyridones by cyclocondensation
[0226] To a mixture of substituted acetic acid (1 mmol) and aminoketone (1 mmol) was added POCl 3 (3ml). The resulting mixture was heated at 70°C for 12 hours under a nitrogen atmosphere. Remove POCl 3 , and the residue was dried under vacuum for 1 h. Then, the residue was dissolved in HOAc (98% acid, 2% water) (2ml) and NH 4 Ac (77mg, 1mmol) was heated at 90°C for 3 hours. The reaction mixture was cooled to room temperature and HOAc was removed. The resulting residue was purified on silica gel using an appropriate solvent system.
Embodiment 2
[0227] Example 2: General method for the synthesis of quinolones and naphthyridones by cyclocondensation
[0228] To a mixture of 2-substituted acetic acid (1.5 mmol) and aminoketone (1 mmol), DIEA (0.7 ml, 4 mmol) in THF (10 ml) was added PyBrop (730 mg, 1.5 mmol). The resulting mixture was stirred overnight at room temperature. If LC / MS and / or TLC of the reaction mixture shows complete formation of the expected fully cyclized product, the solvent is removed and the product is isolated by silica gel chromatography using an appropriate solvent system. If the cyclization reaction was found to be incomplete, toluene (10ml) and piperidine (1ml) were added to the reaction mixture and the resulting mixture was heated at 70°C until complete formation of the expected quinolone was observed by LC / MS. The solvent is then removed and the product isolated by conventional means.
Embodiment 3
[0229] Example 3: 3-(3-Methyl-isoxazol-5-yl)-4-phenyl-1H-[1,5]naphthyridine-2-one
[0230]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com