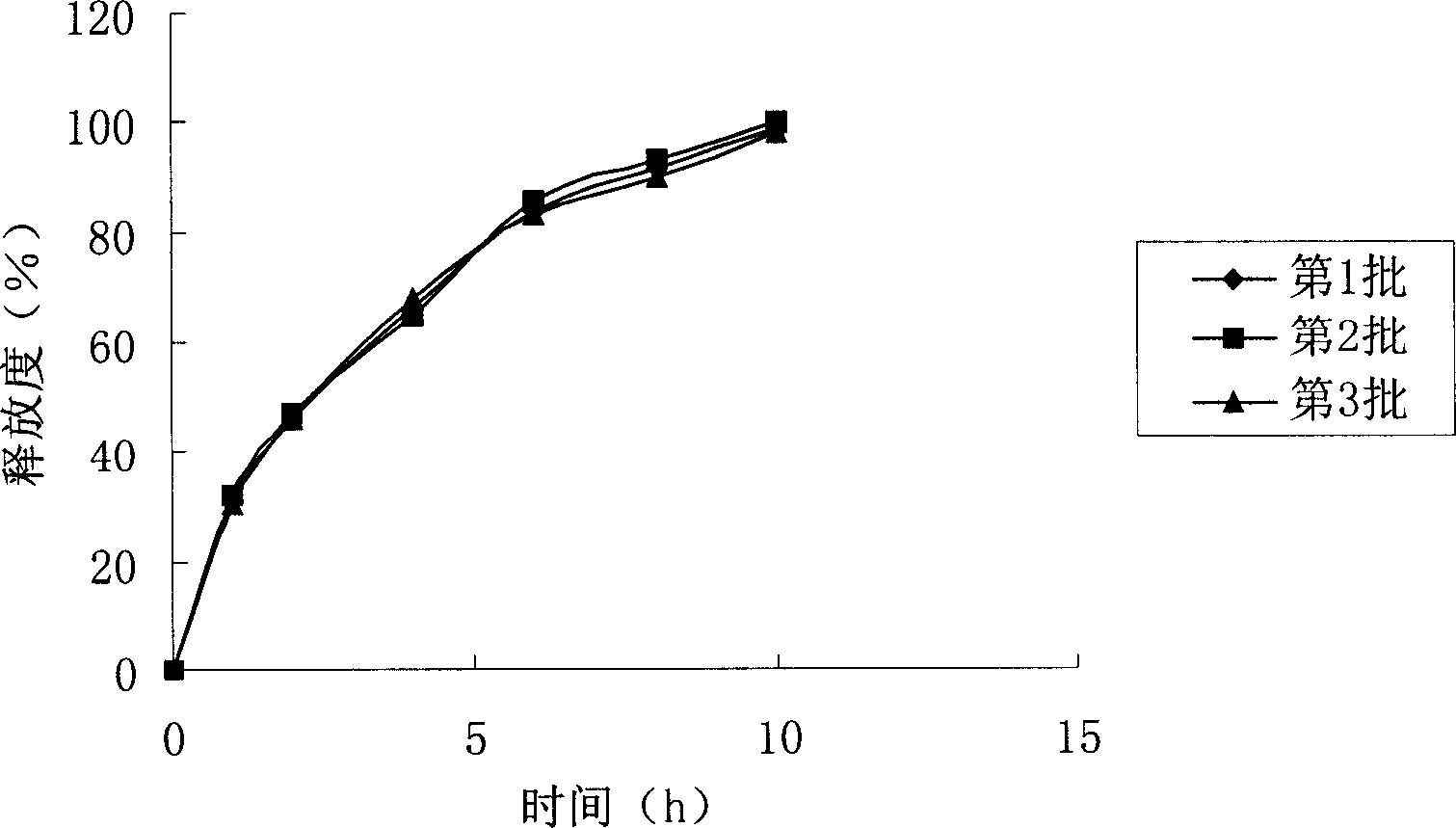
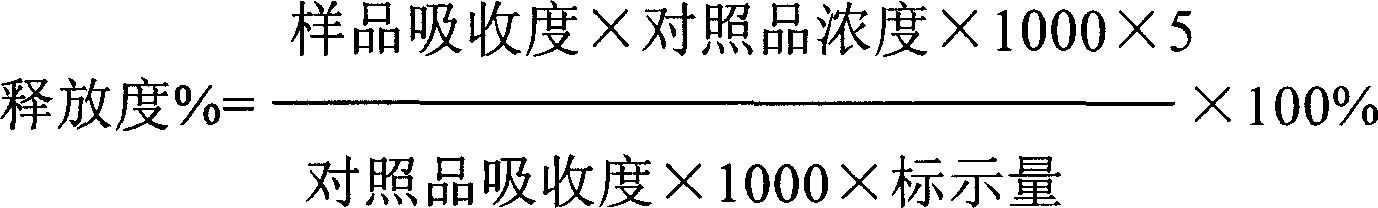Gastrodin slow release preparation
A technology of sustained-release preparations and gastrodin, which is applied in the directions of medical preparations containing active ingredients, drug delivery, organic active ingredients, etc., can solve the problems of poor compliance, unstable blood drug concentration, and frequent taking of ordinary oral preparations, etc. Easy-to-dose results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 gastrodin sustained release coated tablet
[0033] prescription
[0034] Tablet prescription:
[0035] Ingredient Weight
[0036] Gastrodin 75mg
[0037] HPMC-75RT15000 54mg
[0038] HPMC-75RT4000 54mg
[0039] Microcrystalline Cellulose 84.3mg
[0040] 3% PVP in 75% ethanol solution 0.2ml
[0041] Magnesium Stearate 2.7mg
[0042] Coating prescription:
[0043] GS-N type coating powder 8.1mg
[0044] Preparation:
[0045] Chip process:
[0046] (1) Pulverization: take the raw and auxiliary materials of prescription quantity, pass through 80 mesh sieves respectively;
[0047](2) Granulation: mix gastrodin, hypromellose and microcrystalline cellulose, add 3% PVP in 75% ethanol solution as wetting agent to make soft material, pass through a 20-mesh sieve to granulate.
[0048] (3) Drying and sizing: dry the wet granules at 50-60°C, and sieve with 18 meshes.
[0049] (4) Tablet compression: add magnesium stearate to the dry gran...
Embodiment 2
[0055] The preparation of embodiment 2 gastrodin sustained-release tablets
[0056] Prescription: Ingredients Weight
[0057] Gastrodin 75mg
[0058] HPMC-75RT15000 54mg
[0059] HPMC-75RT4000 54mg
[0060] Microcrystalline Cellulose 84.3mg
[0061] 3% PVP in 75% ethanol solution 0.16ml
[0062] Magnesium Stearate 2.7mg
[0063] Preparation method: wet granulate gastrodin, hydroxypropyl methylcellulose, and microcrystalline cellulose, after granulation, add magnesium stearate and mix well, control the tablet weight, and press into tablets to obtain gastrodin sustained-release tablets .
Embodiment 3
[0064] The preparation of embodiment 3 gastrodin sustained-release tablets / capsules
[0065] Recipe: Ingredients Weight
[0066] Gastrodin 75mg
[0067] Sodium Alginate 66mg
[0068] Calcium Alginate 10mg
[0069] Lactose 40mg
[0071] Water (or ethanol aqueous solution) 0.3ml
[0072] Preparation method: fully mix gastrodin, sodium alginate, calcium alginate, and lactose, add appropriate amount of water (or ethanol aqueous solution) to make soft material, dry after granulation, granulate, add magnesium stearate and mix evenly, The weight of the tablet is controlled, and the tablet is obtained immediately. The granules can also be added into hollow capsules after granulation, drying and sizing to obtain gastrodin sustained-release capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com