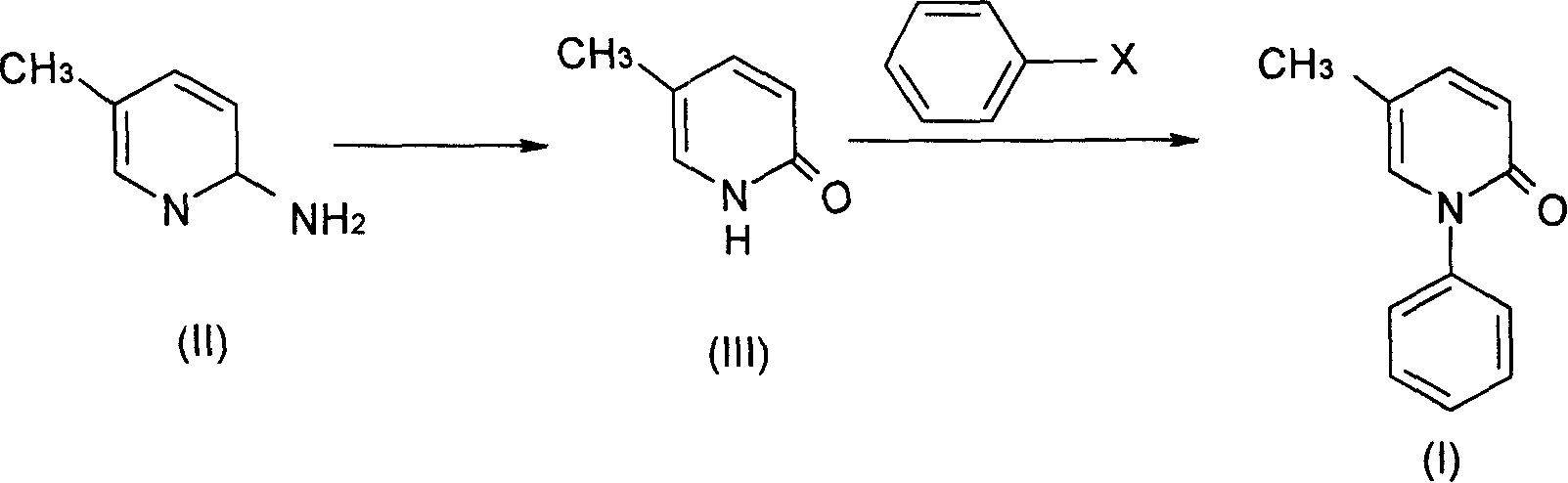
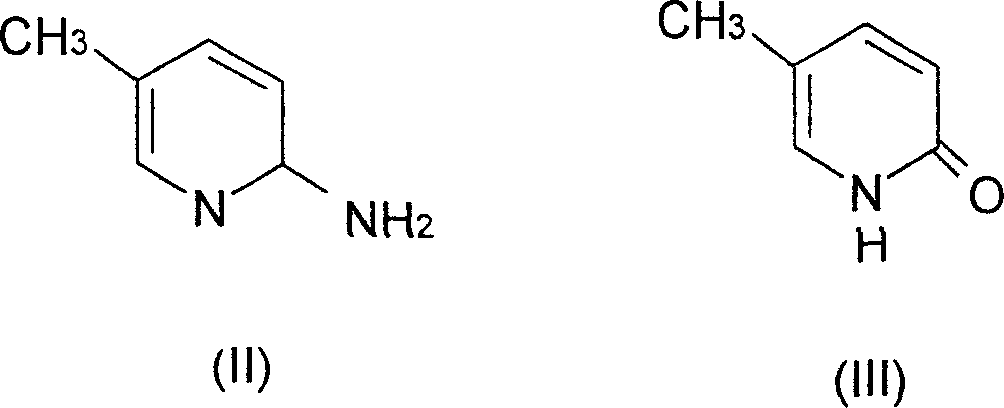
Production of pyriphenanthrenone as anti-fibrosis medicine
A pirfenidone and anti-fibrosis technology, applied in the new field of organic chemical synthesis, can solve the problems of unsuitable industrial production, long preparation method, expensive and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0036] (a).5-Methyl-2(1H)pyridone
[0037] 10.80 g of 2-amino-5-picoline and 250 ml of 5% sulfuric acid were successively added into the three-necked flask, and an aqueous solution of sodium nitrite (12.00 g) was added dropwise at 0-5° C.; then, the reaction solution was heated and hydrolyzed. After completion of the reaction, neutralize with sodium carbonate, concentrate and dry under reduced pressure to obtain a brownish-yellow solid, which is the crude product of 5-methyl-2(1H)pyridone; recrystallize from absolute ethanol to obtain 7.90 g of light yellow crystals, with a yield of 72.5% , mp182-183°C (literature mp181-182°C). IR (cm -1 ): 2842.7, 2840.0 (N-H), 1657.0 (C=O), 1145.1 (C-N). 1HNMR (δppmCDCl3): 2.10 (3H, s, 5-CH3), 6.53 (1H, d, H-3), 7.17 (1H, d, H-4), 7.33 (1H, d, H-6), 13.52 (1H, s, N-H).
[0038] (b). Pirfenidone
[0039] 5.45 g of 5-methyl-2(1H) pyridone, 7.66 g of anhydrous potassium carbonate, 20 g of iodobenzene, and 0.16 g of cuprous chloride were su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com