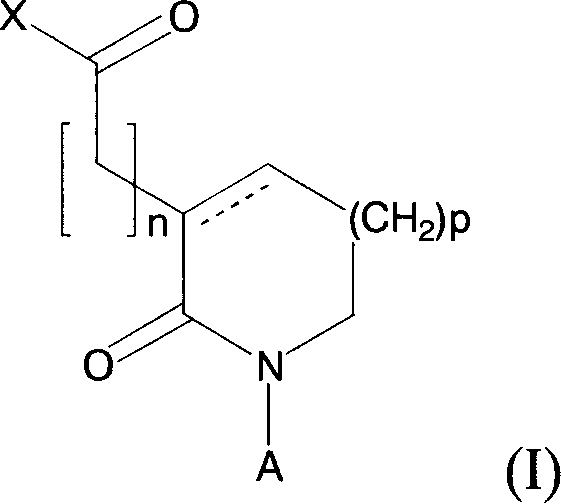
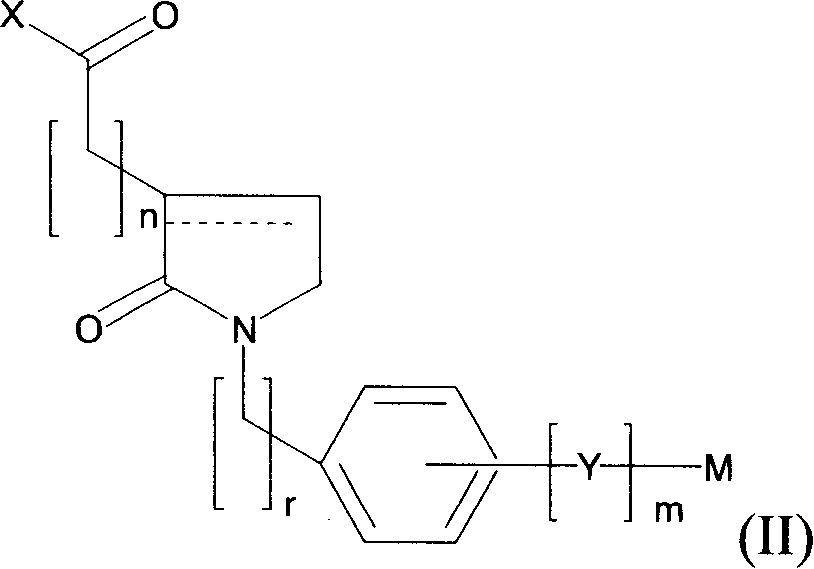
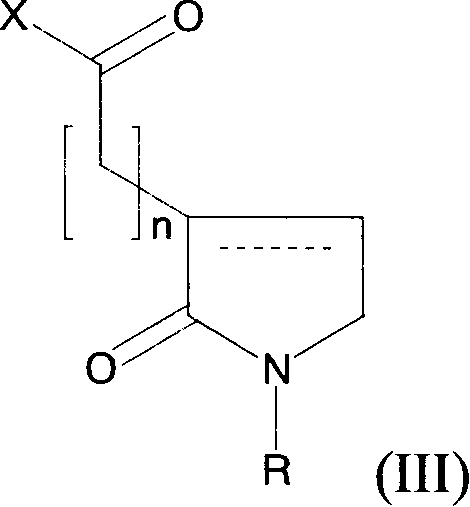
Novel 2-oxo-heterocyclic compounds and the pharmaceutical compositions comprising the same
A compound, oxo technology, applied in physical therapy, organic chemistry, massage auxiliary products, etc., can solve the problems of side effects, limit the use of conventional drugs, can not achieve satisfactory results, and achieve the effect of strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Example 1. Preparation of 3-[1-(2,4-dimethoxybenzyl)-2-oxo-2,5-dihydro-1H-pyrrol-3-yl]-N-hydroxyl propionamide ( 1e)
[0225]
[0226] Step 1. Preparation of Allyl-(2,4-Dimethoxybenzyl)amine (1b)
[0227] Under stirring, 0.32 ml of allyl bromide (3.66 mM) and 0.7 ml of diisopropylethylamine (3.99 mM) were added to 500 mg of 2,4-dimethoxybenzyl dissolved in dichloromethane In the reaction solution of amine (3.33 mM), the solution was left alone at room temperature. After the reaction mixture was neutralized with 10% NaOH solution, the mixture was extracted with chloroform, washed with saturated NaCl solution, washed with MgSO 4 Dry, filter, and concentrate in vacuo. The formed compound was purified by silica gel column chromatography using a solvent mixture of methanol and chloroform (1:9) as eluent to obtain 276 mg of allyl-(2,4-dimethoxybenzyl)amine (1b) ( Yield: 40%).
[0228] 1 H-NMR (300MHz, CDCl 3 )δ7.12(d, J=8.1Hz, 1H), 6.44-6.39(m, 2H), 5.99-5.86(m, 1H)...
Embodiment 2
[0238] Example 2. Preparation of 3-(1-benzyl-2-oxo-2,5-dihydro-1H-pyrrol-3-yl)-N-hydroxyl-propionamide (2e)
[0239] 3-(1-Benzyl-2-oxo-2,5-dihydro-1H-pyrrol-3-yl)-N-hydroxy-propionamide (2e) was prepared in a similar manner to that described in Example 1 above 1 (see Table 1).
Embodiment 3
[0240] Example 3. Preparation of N-hydroxy-3-(2-oxo-1-phenethyl-2,5-dihydro-1H-pyrrol-3-yl)-propionamide (3e)
[0241] N-Hydroxy-3-(2-oxo-1-phenethyl-2,5-dihydro-1H-pyrrol-3-yl)-propionamide (3e) was prepared in a similar manner to that described in the above examples 1 (see Table 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com