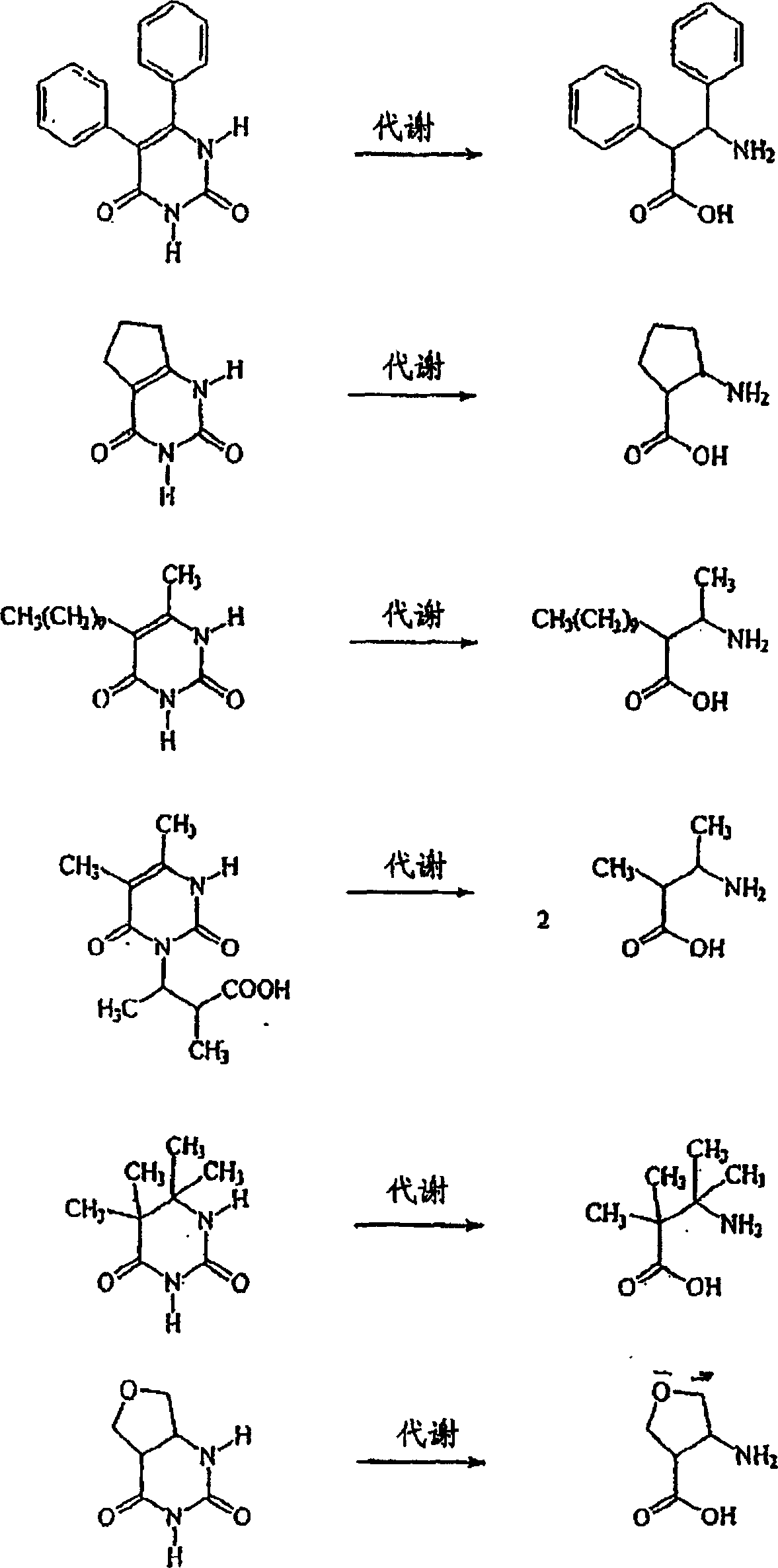
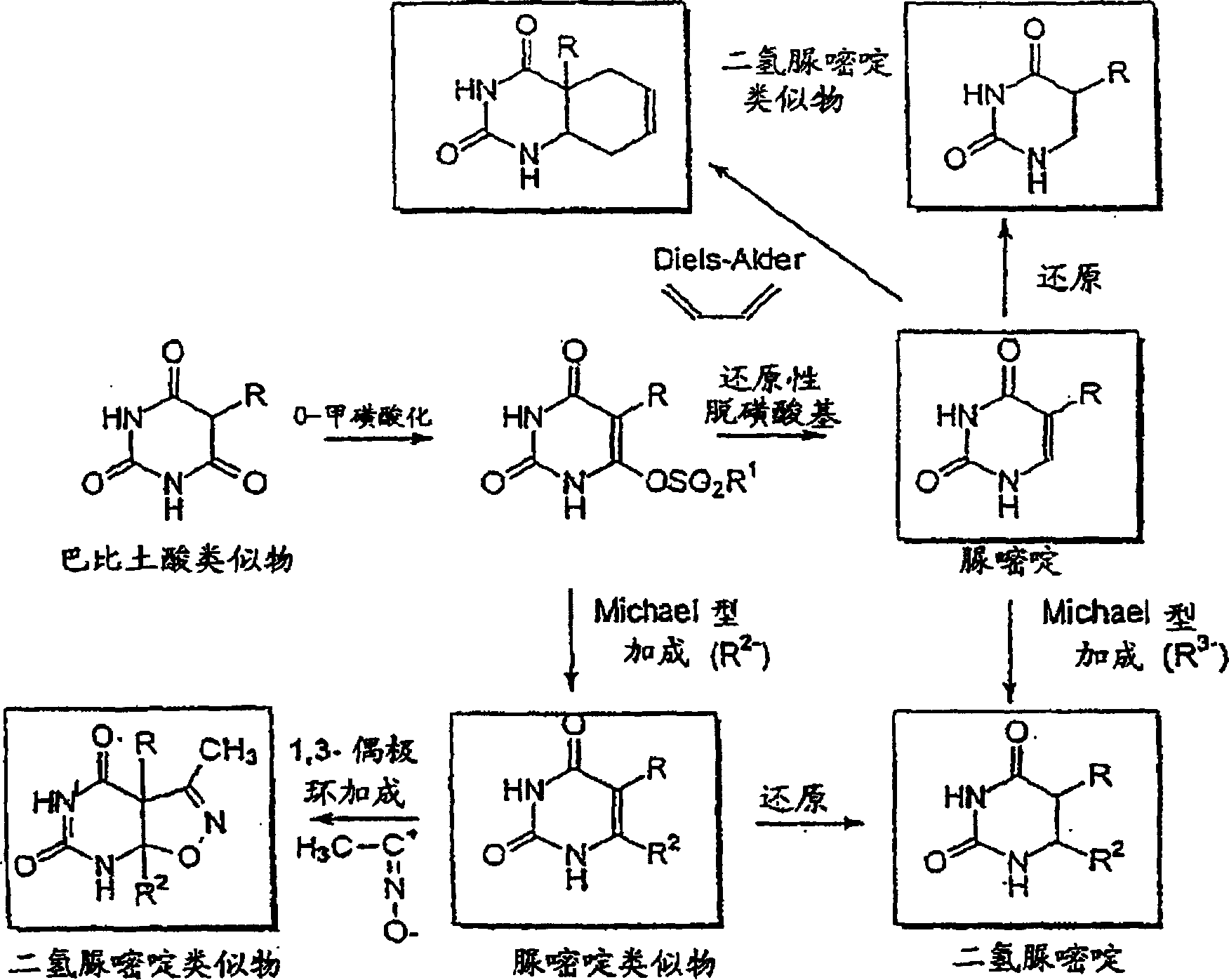
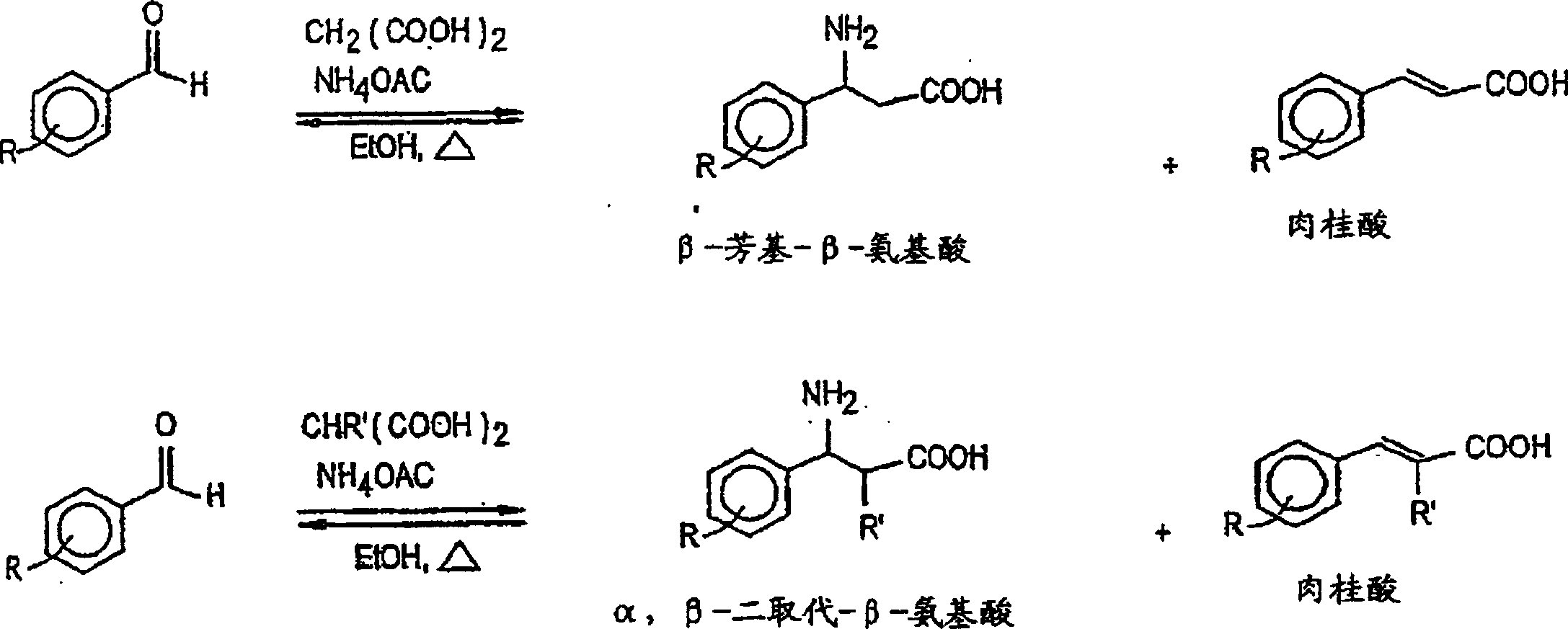
Anti-epileptogenic agents
A technology of epilepsy and compounds, applied in the field of anti-epileptic agents, can solve problems such as no epilepsy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0237] The preparation method of III.β-amino anion compound
[0238] The invention also provides a preparation method of the β-amino anion compound.
[0239] In one embodiment, the present invention includes a process for the preparation of a β-amino anionic compound represented by the following formula (Formula VI):
[0240] or
[0241] Formula VI
[0242] Wherein the dashed line represents an optional single / double bond (E- or Z-configuration); R 2 and R 3 each independently hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, alkylcarbonyl, arylcarbonyl, alkoxycarbonyl, or aryloxycarbonyl; or R 2 and R 3 Joined with the attached nitrogen to form an unsubstituted or substituted heterocyclic ring with 3-7 atoms in the heterocyclic ring; R 4 and R 5 each independently hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, alkylcarbonyl, arylcarbonyl, alkoxycarbonyl, aryloxycarbonyl, amino, hydroxyl, cyano, alkoxy, aryl Oxy, carboxy, alkoxyca...
Embodiment 1
[0279] Example 1: Identification of Compounds Based on Pharmacophore Models
[0280] A pharmacophore model was developed incorporating the structural parameters and properties of two different classes of compounds: (1) GABA uptake receptor inhibitors, and (2) NMDA receptor co-antagonists.
[0281] Previous models (Murali Dhar et al. (1994) J. Med. Hem. 37: 2334, Falch and Krogsgaard-Larson (1991) Eur. J. Med. Chem. 26: 69, N'Goka (1991) J. Med.Chem 34:2547) suggests that GABA absorption inhibitors should include:
[0282] i) Amine functionality (preferably secondary amine)
[0283] ii) Carboxyl functional group
[0284] iii) lipophilic groups, preferably aromatic
[0285] iv) Electron-rich functional group (double bond or oxygen) located between amine and lipophilic group
[0286] v) Two-carbon chain length between amine functional group and double bond or oxygen atom
[0287] Other previous models for antagonists of the glycine co-agonist site of the NMDA receptor comple...
Embodiment 2
[0302] Example 2: In vivo evaluation of the pharmacological efficacy of candidate compounds for inhibiting epileptogenesis
[0303] The anti-seizure activity and neurotoxicity of two sets of candidate analogs were tested in vivo. A seizure model was established using adult male Sprague-Dawley rats following the guidelines of the Canada Council on Animal Care and under the supervision of the Queen's University Animal Ethics Committee. This protocol has been adopted from previous work by Turski et al. (1984) Brain Res. 321:237. Test compounds were administered by intraperitoneal (i.p.) injection at a dose of 100 mg / kg. Seizures were induced 20 minutes after i.p. administration of pilocarpine hydrochloride (350 mg / kg). Protection was defined as the absence of chronic convulsions during the 30-minute observation period following pilocarpine administration. Using this assay, compounds 1, 2, 3, 5, 8, 10, 11, 13, A1, A4, A5, A11, A13, A14, A15, A16, A21, A26, A28, A29 and A31 exhi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com