Liposomal glucocorticoids
A technology of glucocorticoids and liposomes, which is applied in liposome delivery, endocrine system diseases, bone diseases, etc., can solve the problems of low stability of preparations, unpopularity, and reduced half-life of circulation, etc. Chemicals and pharmaceuticals, the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Preparation of liposomes filled with dexamethasone phosphate
[0128] A mixture of 50 mol% DPPC, 10 mol% DPPG and 40 mol% Chol was dissolved in chloroform and then completely dried in a rotary evaporator under vacuum.
[0129] Add dexamethasone phosphate solution (25 mg / ml dexamethasone phosphate in 10 mM HEPES and 150 mM NaCl, pH 7.5) into the liquid film in an amount capable of forming a 100 mM suspension. Next, the suspension was hydrated in a water bath at 50° C. for 45 minutes with shaking, and then treated in an ultrasonic bath for 5 minutes. Thereafter, the solution was frozen.
[0130] After thawing, the liposomes were repeatedly extruded through a membrane with a pore width of 400 nm. Uncoated dexamethasone phosphate was removed by gel filtration.
Embodiment 2
[0132] Determination of Dexamethasone Phosphate Release
[0133]Liposomes were prepared as in Example 1, and diluted with buffer (10 mM HEPES, 150 mM NaCl, pH 7.5) to a concentration of 12 mM lipid. Next, incubate at 37°C. Aliquots of this incubation solution were promptly removed at defined time points (see table below). The content of dexamethasone phosphate in aliquots was determined using RP-HPLC.
[0134] Table 1
[0135] The release of dexamethasone phosphate as a percentage of the coated amount at different times after incubation in buffer at 37°C
[0136] Preparation Lipid mol% 0 hours 24 hours 120 hours
[0137] A DPPC / DPPG / Chol 50:10:40 1.1 6.0 7.3
[0138] Liposomes showed sufficient stability during the assay. In 5 days, less than 8% of the coated active substance was released.
Embodiment 3
[0140] Uses of liposomal dexamethasone phosphate
[0141] According to Buchner method ("Behandlung der antigeninduzierten Arthritisder Ratte mit Anti-Makrophagenprinzipien und monoklonalen Anti-CD4Antikrpern", Ph.D.thesis 1996, Friedrich-Alexander University Erlangen-Nuremberg, Germany, p.129), in test animals (rats ) to produce antigen-induced arthritis.
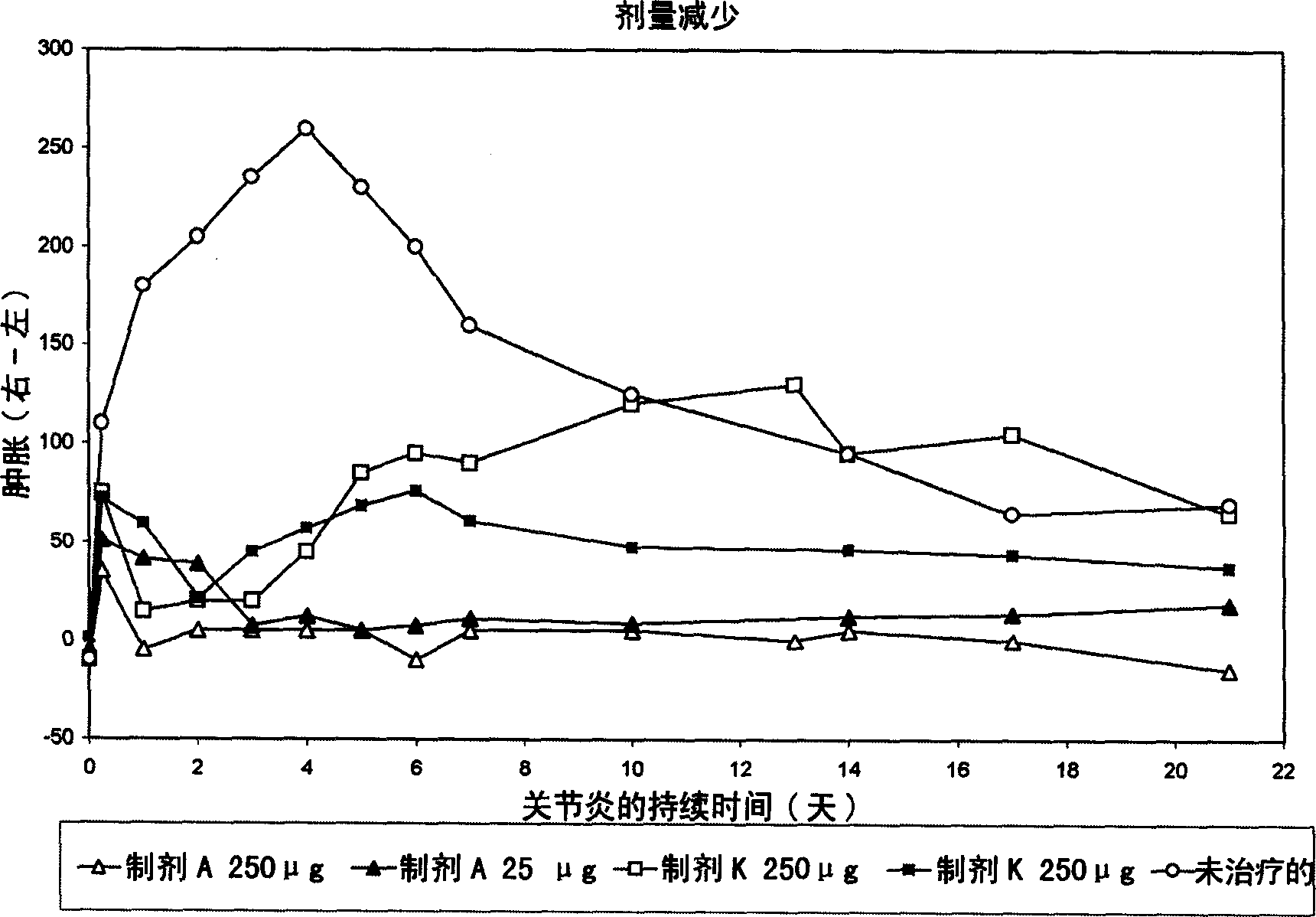
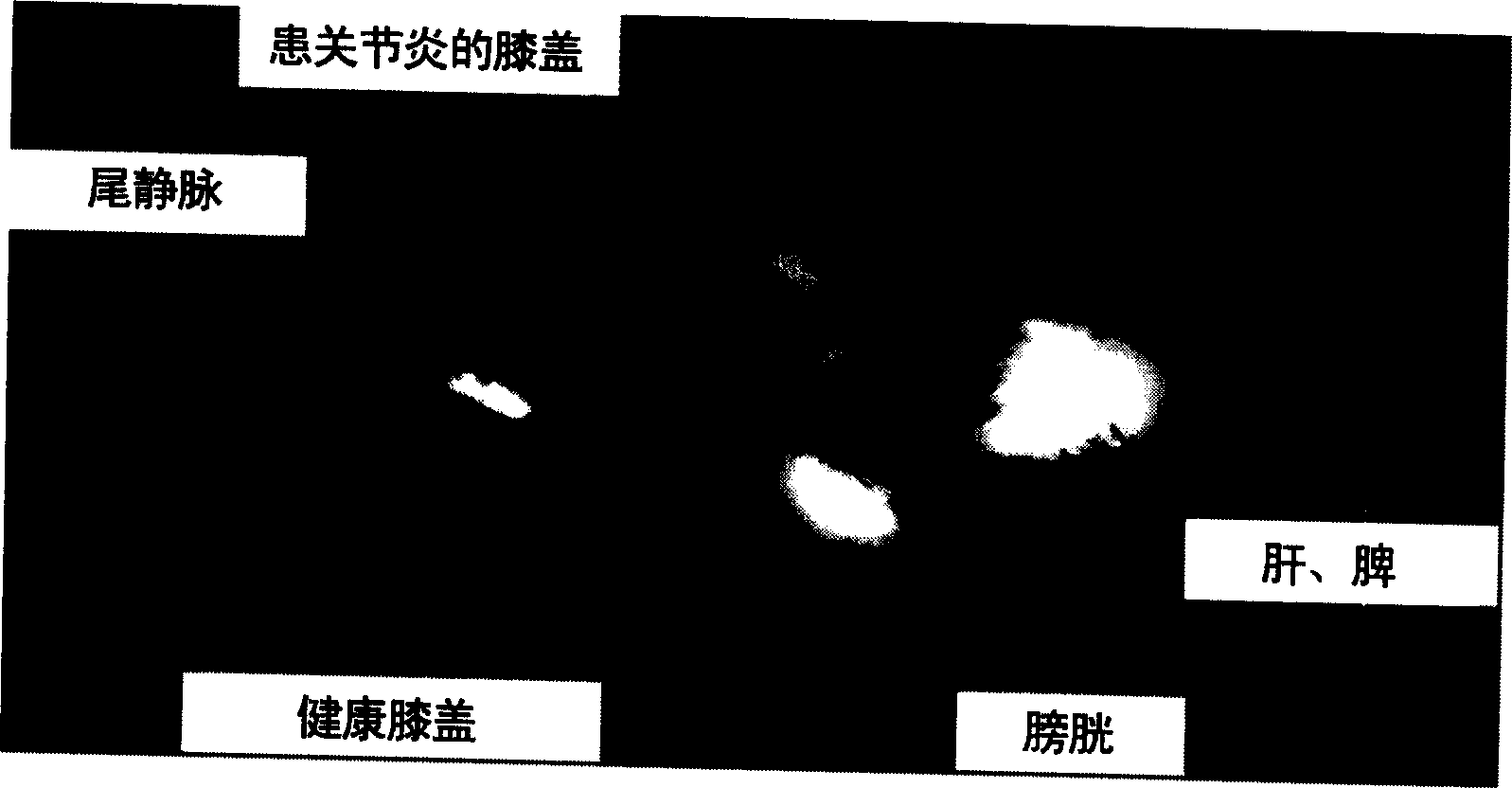
[0142] Antigen was injected into the synovial fluid of the right knee by intra-articular injection according to the Buchner method, and arthritis was induced on day 0. Arthritic animals were treated with intravenous injection of liposomal dexamethasone phosphate (Formulation A, 3.75 mg / kg) at 6, 24 and 48 hours after arthritis induction. Free dexamethasone phosphate (formulation K, also 3.75 mg / kg) and saline solution (saline) served as controls. The following parameters were used to establish the effect of sample administration.
[0143] - Determination of joint swelling (cf., figure 1 )
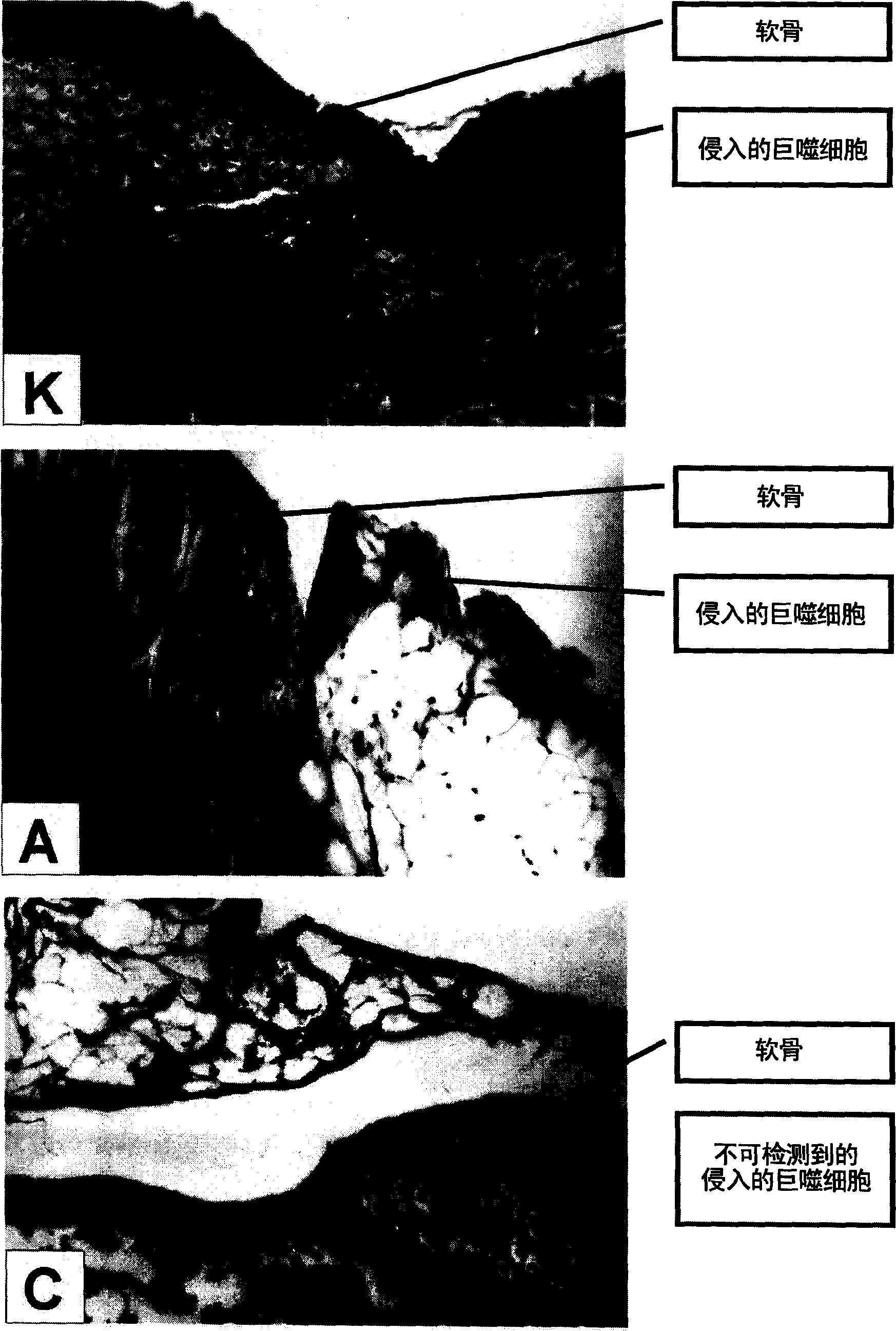
[0144] - parameters of hist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com