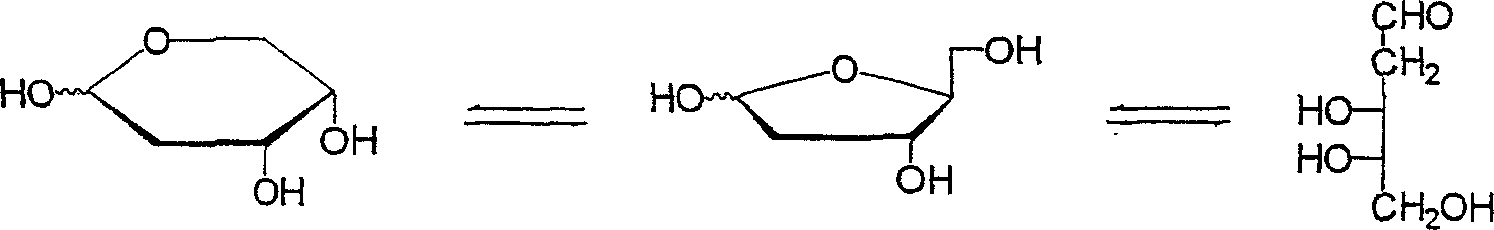
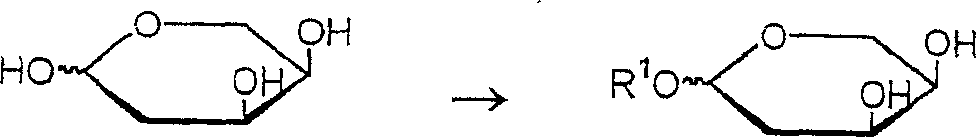Method for producing a 2-deoxy-L-ribose
一种核糖、烷基的技术,应用在2-脱氧-L-核糖的制备领域,能够解决使用昂贵、高毒性试剂、艰难分离和纯化等问题,达到容易分离和纯化的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0047] Protection: Preparation of 2-deoxy-1-O-butyl-D-ribose
[0048] To cooled butanol containing 3% HCl (56.4 mL), 2-deoxy-D-ribose (robse) (10 g) was added and stirred at -2°C for 16 hours. Keeping the temperature below 10°C, the reaction mixture was neutralized with triethylamine and stirred at 20°C to 25°C. The mixture was filtered and washed with acetone (20 mL). The combined filtrate and washing solution were concentrated and used for further reaction without purification.
Embodiment approach 2
[0050] Activation of the 3-4-OH group: Preparation of 2-deoxy-1-O-butyl-3,4-bis(p-toluenesulfonyl)-D-ribose
[0051]Below 30°C, to the solid of 2-deoxy-1-O-butyl-D-ribose obtained in Embodiment 1, gradually add pyridine (36 mL), p-toluenesulfonyl chloride, and at 27°C to 30°C The mixture was stirred for 20 hours. The reaction mixture was heated to 75±2°C and stirred for 2 hours. After the reaction was completed, the mixture was cooled to 15°C to 20°C, and pure water (30 mL) was added, and the resulting mixture was extracted twice with ethyl acetate (30 mL). The extract was concentrated, and ethanol and isopropanol were added. The solution was cooled, filtered and dried to give 2-deoxy-1-O-butyl-3,4-di-(p-toluenesulfonyl)-D-ribose as a solid (23 g).
Embodiment approach 3
[0053] Conversion from D-form sugars to L-form sugars: 2-deoxy-1-O-butyl-3-benzoyl-L-ribose and 2-deoxy-1-O-butyl-4-benzoyl- Preparation of L-ribose
[0054] To the compound (20 g) obtained in Embodiment 2, were added n-butanol (7 mL), water (4.4 mL), N,N-dimethylformamide (27.6 mL) and potassium benzoate (21.2 g) , and the reaction mixture was heated to 115° C. for 8 hours. After the reaction mixture was concentrated, water and ethyl acetate were added to the separated organic and aqueous layers. After concentration of the organic layer, the residue was mixed with water and evaporated again to effectively concentrate the dimethylformamide. The residue was used for the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com