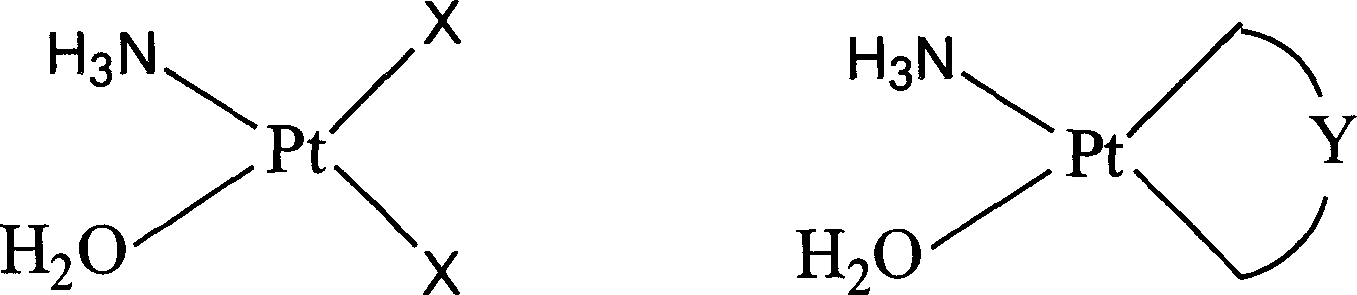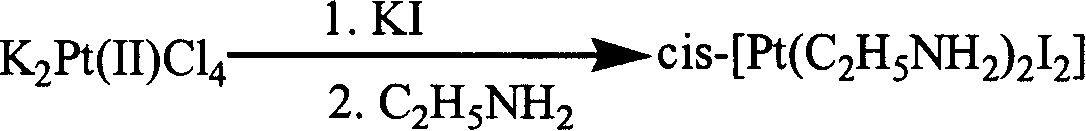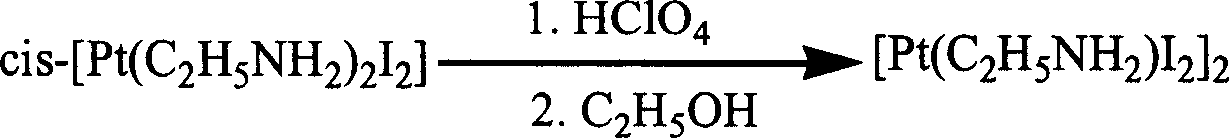Antineoplastic platinum complex
A technology of platinum complexes and compounds, applied in the field of platinum complexes of new anti-tumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Complex (a)[Pt(NH 3 )(H 2 O)(OOC) 2 ] Preparation:
[0082] ①cis-[Pt(C 2 h 5 NH 2 ) 2 I 2 ] Preparation of (i): Dissolve potassium chloroplatinite in water, add potassium iodide solution at an injection rate of 15 ml / min under agitation, react at 25° C. for 40 minutes, filter, and under agitation, Add ethylamine to the filtrate at an injection rate of 20 ml / min, react at 25°C for 30 minutes, filter the precipitate obtained by washing with water, ethanol and ether in sequence, then dry under vacuum conditions, potassium chloroplatinite: Potassium iodide: ethylamine=1mmol: 10mmol: 2.5mmol; ②[Pt(C 2 h 5 NH 2 ) I 2 ] 2 Preparation of (ii): Add perchloric acid and ethanol solution to the precipitate obtained in step ① respectively, stir for 40 hours at 50°C, filter, and wash the precipitate obtained by filtering with an ethanol-water mixed solvent to obtain the Platinum complex, vacuum drying then, the precipitate of step 1. gained: perchloric acid: ethanol=1mmo...
Embodiment 2
[0084] Complex (b)[Pt(NH 3 )(H 2 O)(OOC-C 6 h 4 -p-OCH 3 ) 2 ] Preparation:
[0085] The steps for preparing the precursor complex are as described in Example 1 ①, ② and ③, ④ add silver carbonate, p-methoxybenzoic acid and an appropriate amount of methanol solution to the precipitate obtained in step ③, and at 25 ° C, avoid Lightly stirred for 46 hours, filtered, and the precipitate obtained by filtering was washed with water and ether respectively, and then vacuum-dried, the precipitate obtained in step ③: silver carbonate: p-methoxybenzoic acid=1mmol: 0.98mmol: 2.2mmol;
Embodiment 3
[0087] Complex (c)[Pt(NH 3 )(H 2 O)(OOC-C 6 h 5 ) 2 ] Preparation:
[0088] The steps for preparing the precursor complex are as described in Example 1 ①, ② and ③, ④ add silver carbonate, benzoic acid and an appropriate amount of methanol solution to the precipitate obtained in step ③, and stir for 40 hours at 35 °C in the dark , filtered, the precipitate obtained by filtering was washed with water and ether respectively, then vacuum-dried, the precipitate obtained in step 3.: silver carbonate: benzoic acid=1mmol: 0.96mmol: 2.1mmol;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com