Two step preparation of dinitrotoluene method
A technology for dinitrotoluene and nitrotoluene, applied in the field of two-step preparation of dinitrotoluene, can solve the problems of high energy consumption, inability to prepare DNT, high cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
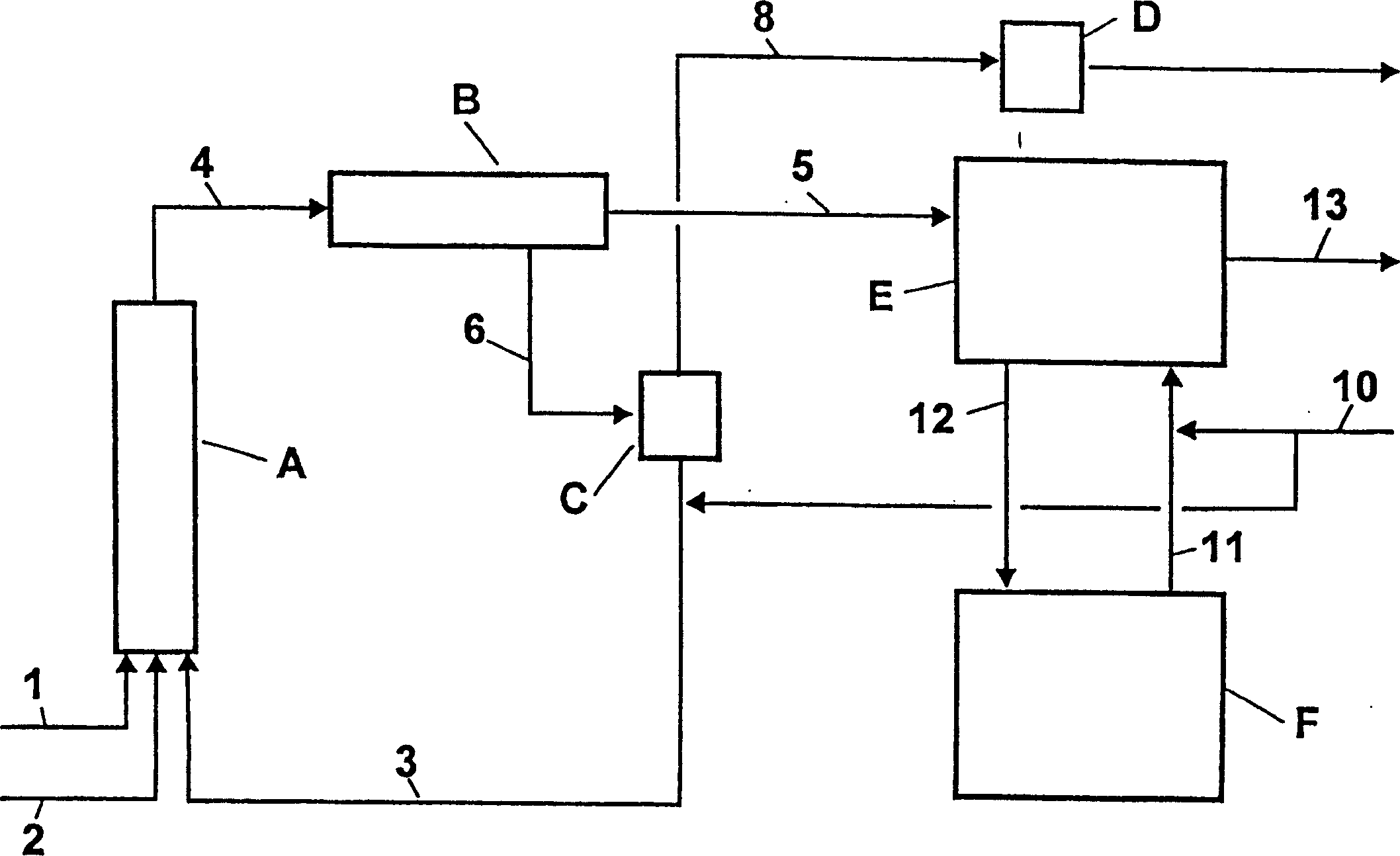
[0047] use figure 1 device shown. The temperature and composition of the streams are shown in Table 1.
[0048] In the first step of the adiabatic operation, 50.6 kg / h of toluene (stream 1), 63.0 kg / h of 68% by weight nitric acid (stream 2) and 1066.6 kg / h of 76.8% by weight were thoroughly mixed at the inlet of reactor A The spent concentrated acid (stream 3). The dimensions of reactor A are as follows: length (L) = 5 m, diameter (D) = 25 / 80 mm. In a tubular reactor equipped with perforated discs for redispersion, the temperature was raised to 131°C. With an average residence time of 15 minutes, the reaction mixture (stream 4) was separated in phase separator B into 78.1 kg / h organic phase (stream 5) and 1102.1 kg / h acidic aqueous phase (stream 6). The 74.5% by weight of spent acid was concentrated to 76.8% by weight of sulfuric acid in a flash evaporator C operated at 40 mbar. To this end, a small amount of secondary heat is supplied via a heat exchanger at the bottom o...
Embodiment 2
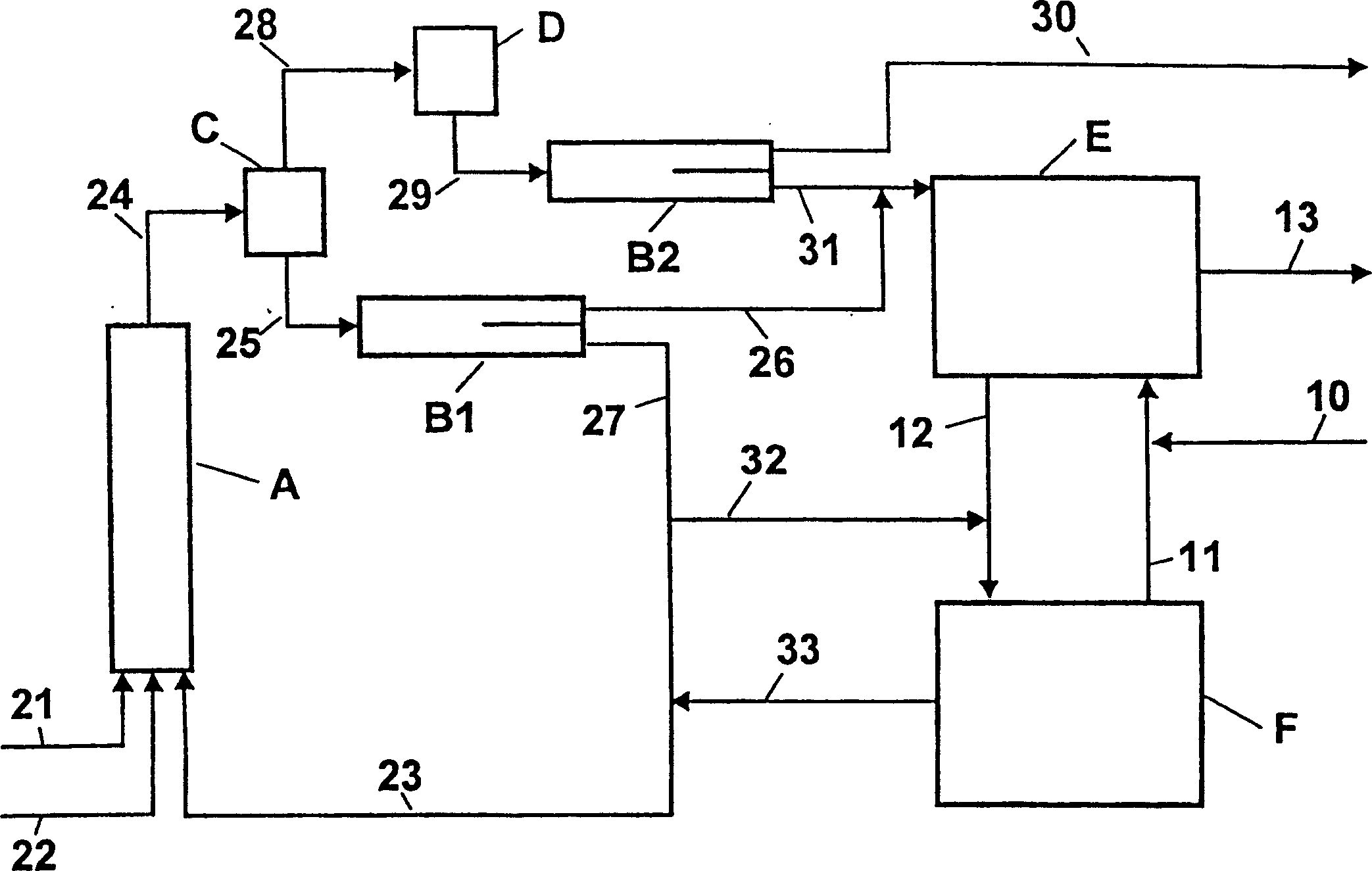
[0053] use figure 2 device shown. The temperature and composition of the streams are shown in Table 2.
[0054] In the first step of the adiabatic operation, 50.6 kg / h of toluene (stream 21), 62.9 kg / h of 68% by weight nitric acid (stream 22) and 736.0 kg / h of 78.6% by weight were thoroughly mixed at the inlet of reactor A of concentrated spent acid (stream 23). The dimensions of reactor A are as follows: length (L) = 8 m, diameter (D) = 80 mm. In a tubular reactor equipped with perforated discs for redispersion, the temperature was raised to 138°C. The reaction mixture (stream 24) is depressurized through nozzles into flasher C operated at 40 mbar, where part of the water and organic components are evaporated. The vapor is then condensed in heat exchanger D. No deposits were formed in the vapor condenser D despite cooling with cold water at 18°C. In phase separator B2, the vapor condensate (stream 29) is separated into an aqueous phase (21.3 kg / h, stream 30) and an org...
Embodiment 3
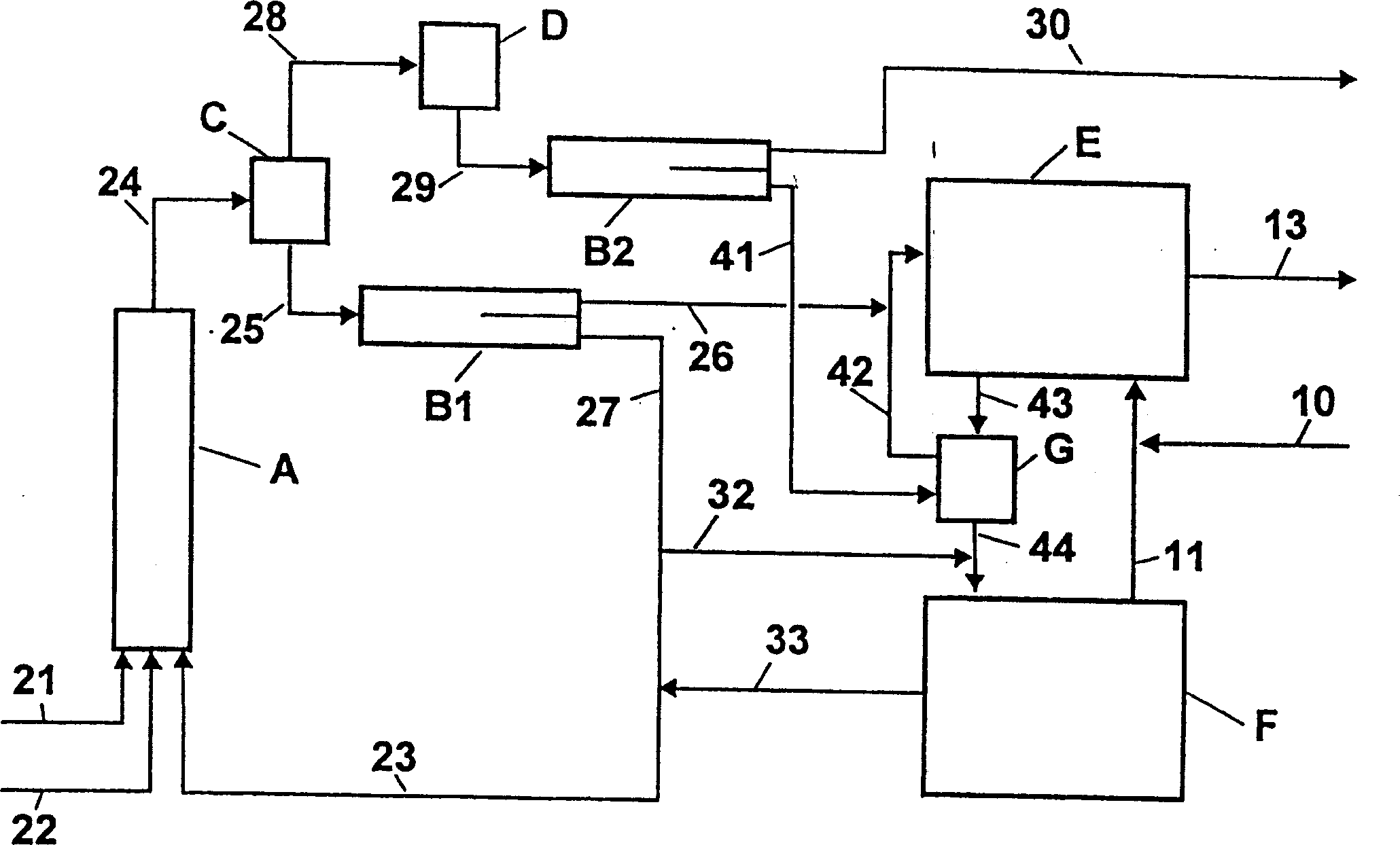
[0059] use image 3 device shown. The temperature and composition of the streams are shown in Table 3.
[0060] In the first step of the adiabatic operation, 50.6 kg / h of toluene (stream 21), 63.0 kg / h of 68% by weight nitric acid (stream 22) and 1306.6 kg / h of 82.4% by weight were thoroughly mixed at the inlet of reactor A of concentrated spent acid (stream 23). The dimensions of reactor A are as follows: length (L) = 5 m, diameter (D) = 80 mm. In a tubular reactor equipped with perforated discs for redispersion, the temperature was raised to 132°C. The reaction mixture (stream 24) is depressurized through nozzles into flasher C operated at 40 mbar, where part of the water and organic components are evaporated. The vapor is then condensed in heat exchanger D. No deposits were formed in the vapor condenser D despite cooling with cold water at 18°C. In phase separator B2, the vapor condensate (stream 29) is separated into an aqueous phase (18.3 kg / h, stream 30) and an org...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com