Stable recombination human alpha interferon liquid preparation and productive process thereof
A liquid preparation, alpha interferon technology, applied in the field of recombinant human alpha interferon liquid preparation, can solve the problems of unsatisfactory protective agent and thickener, affecting drug use, unfavorable preservation, etc., achieving high clinical application value and reliable curative effect , the effect of prolonging the adhesion time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0023] Example 1
[0024] formula:
[0025] Recombinant human α2a interferon 3 million IU / ml.
[0026] Sodium chloride 0.85g
[0027] Methylparaben 0.03g
[0028] Propylparaben 0.02g
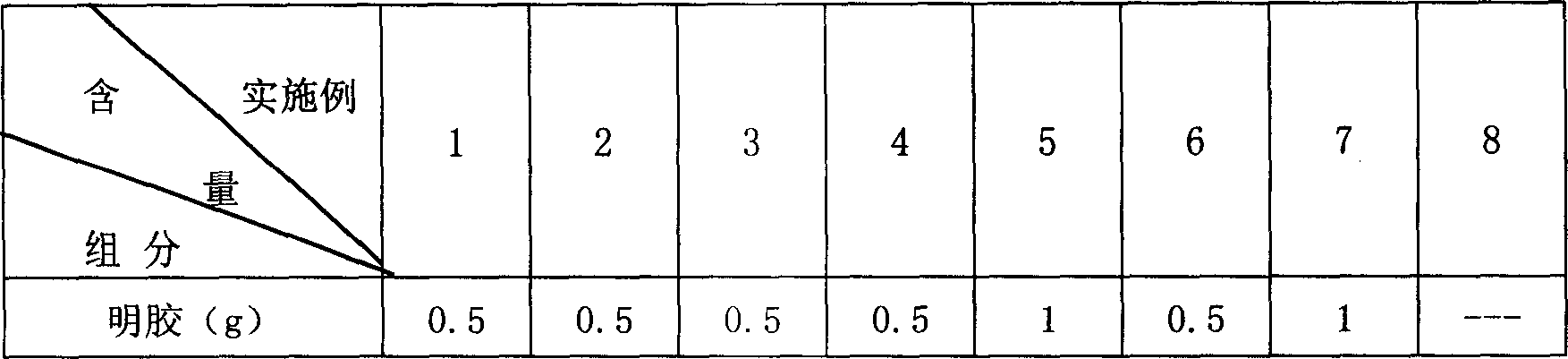
[0029] Gelatin 0.5g
[0030] Sodium Hyaluronate 0.02g
[0031] EDTA-Na 0.005g
[0032] Tween-80 1ml
[0033] Human Albumin 20% 5ml
[0034] Add water for injection to 100ml
[0035] Preparation Process:
[0036] 1. Preparation:
[0037] Solution A: Take sodium chloride, methylparaben, propylparaben, EDTA-Na, and Tween-80 according to the formula, put them in a container, add 70ml of water for injection, autoclave at 121°C for 30 minutes, and set aside.
[0038] Solution B: Slowly add the prescribed amount of sodium hyaluronate into 10ml of cooled water for injection, shake the container while adding, and let it stand overnight to fully dissolve.
[0039] Solution C: Add the gelatin in the prescribed amount into a container with accurate scale, add 15ml of water for injection to fully...
Example Embodiment
[0042] Example 2
[0043] formula:
[0044] Recombinant human α2a interferon 300IU / ml.
[0045] Sodium chloride 0.85g
[0046] Methylparaben 0.03g
[0047] Propylparaben 0.02g
[0048] Gelatin 0.5g
[0049] EDTA-Na 0.005g
[0050] Human Albumin 20% 5ml
[0051] Add water for injection 100ml
[0052] Preparation Process:
[0053] 1. Preparation:
[0054] Liquid A: Take sodium chloride, methylparaben, propylparaben, and EDTA-Na according to the formula, put them in a container, add 70ml of water for injection, autoclave at 121°C for 30 minutes, and set aside.
[0055] Liquid B: Add the gelatin in the prescribed amount into a container with accurate scale, add 15ml of water for injection to fully swell, autoclave at 121°C for 30 minutes, and set aside.
[0056] 2. Preparation of alpha interferon liquid preparation:
[0057] Mix the alpha interferon stock solution, human serum albumin, and the above-prepared liquid A, then sterilize and filter it into liquid B with a 0....
Example Embodiment
[0058] Example 3
[0059] formula:
[0060] Recombinant human α2a interferon 100IU / ml.
[0061] Sodium chloride 0.85g
[0062] Methylparaben 0.03g
[0063] Propylparaben 0.02g
[0064] Gelatin 0.5g
[0065] Sodium Hyaluronate 0.02g
[0066] Human Albumin 20% 5ml
[0067] Add water for injection to 100ml
[0068] 1. Preparation:
[0069] Liquid A: Take sodium chloride, methylparaben, and propylparaben according to the formula, put it in a container, add 70ml of water for injection, autoclave at 121°C for 30 minutes, and set aside.
[0070] Solution B: Slowly add the prescribed amount of sodium hyaluronate into 10ml of cooled water for injection, shake the container while adding, and let it stand overnight to fully dissolve.
[0071] Solution C: Add the gelatin in the prescribed amount into a container with accurate scale, add 15ml of water for injection to fully swell, and then autoclave at 121°C for 30 minutes, and set aside.
[0072] 2. Preparation of alpha interferon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com