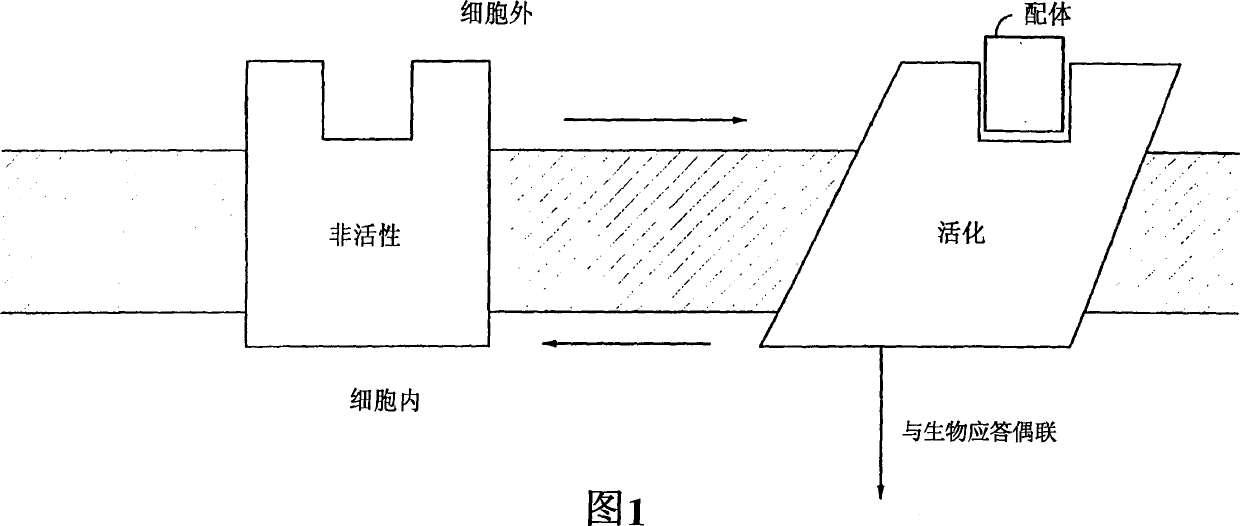
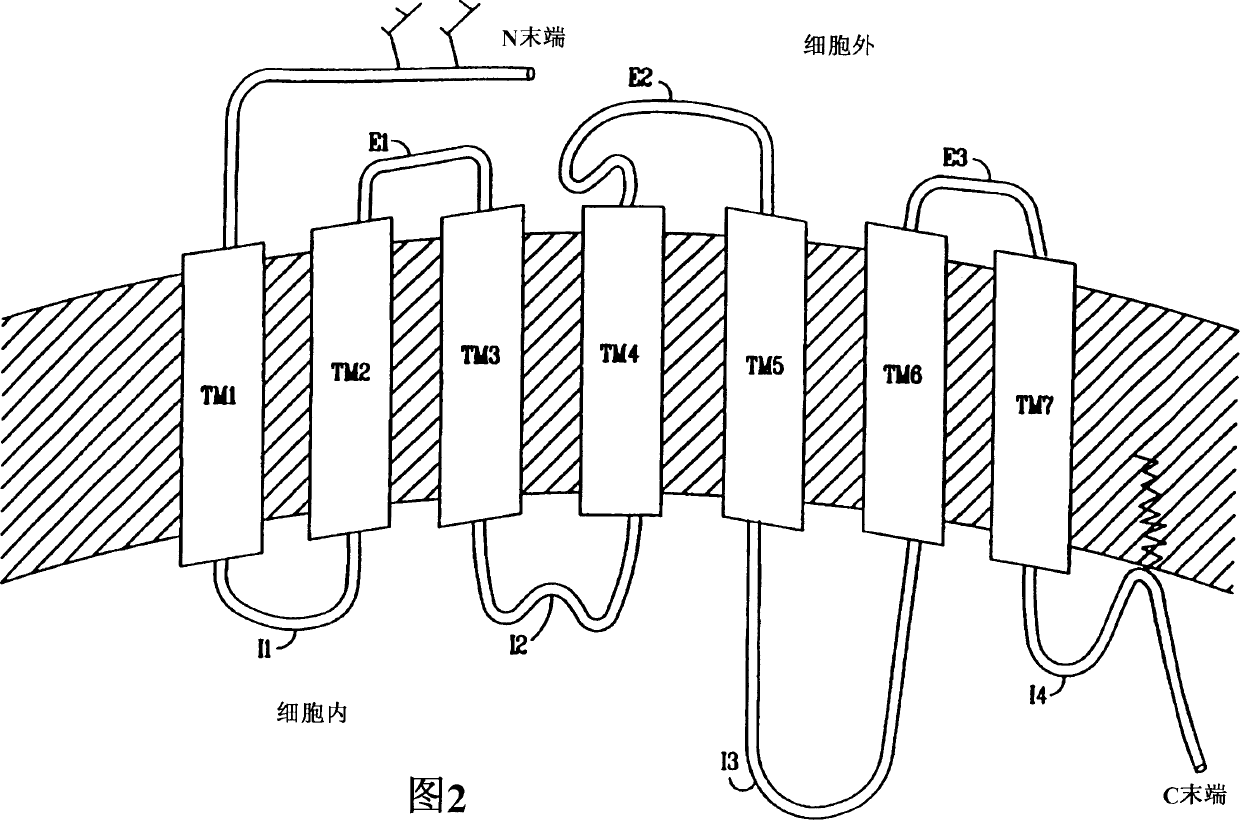
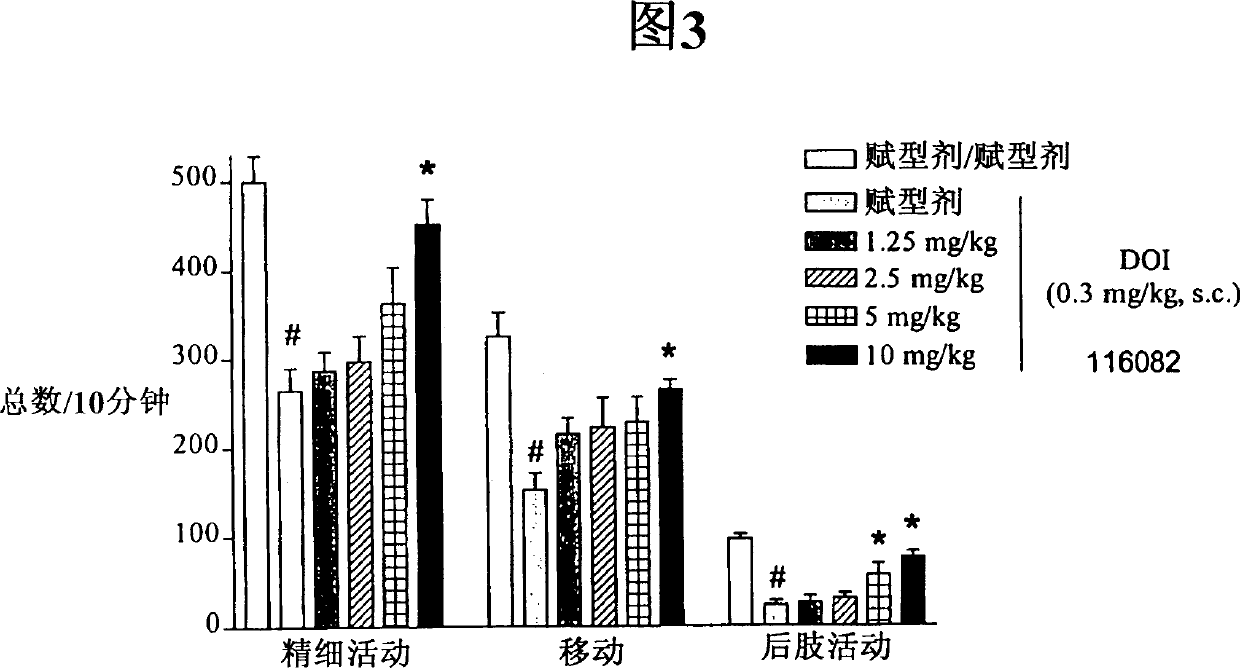
Pyrazole derivatives which modulate human 5-serotonin receptors
A compound and composition technology, applied in the field of small molecule regulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Non-endogenous, constitutively activated human 5-HT receptor 5-HT 2C and 5-HT 2A production
[0057] A. Constitutively active 5-HT 2C Construction of receptor cDNA
[0058] 1. Endogenous human 5-HT 2C
[0059] Encodes endogenous human 5-HT 2C The cDNA of the receptor was obtained from human brain polyadenylic acid (poly-A + ) obtained from RNA. 5' and 3' primers were derived from the 5' and 3' untranslated regions and contained the following sequences:
[0060] 5'-GACCTCGAGGTTGCTTAAGACTGAAGCA-3' (SEQ.ID.NO.: 1),
[0061] 5'-ATTTCTAGACATATGTAGCTTGTACCGT-3' (SEQ. ID. NO.: 2).
[0062] Using TaqPlus TM Precision polymerase (Stratagene) or rTth TM Polymerase (PerkinElmer) and the buffer system provided by the manufacturer were used for PCR, each primer was 0.25 μM, and each of the four nucleotides was 0.2 mM. The cycle conditions were 94°C for 1 minute, 57°C for 1 minute, and 72°C for 2 minutes, and the cycle was 30 times. The 1.5 kb PCR fragment was digested w...
Embodiment 2
[0114] receptor expression
[0115] A.pCMV
[0116] Although a variety of expression vectors are available to those skilled in the art, the most preferred vector for use with the endogenous and non-endogenous receptors discussed in the present invention is pCMV. This vector was deposited with the American Type Culture Collection (ATCC) on October 13, 1998 (10801 University Blvd., Manassas, VA 20110-2209 USA) in accordance with the provisions of the Budapest Treaty on the International Recognition of Deposits of Microorganisms for the Purposes of Patent Procedure. The DNA was tested by the ATCC and certified viable. The ATCC has assigned the following accession number to pCMV: ATCC #203351. See Figure 8.
[0117] B. Transfection Procedure
Embodiment 3
[0120] Protocol: GTP Membrane-Bound Scintillation Proximity Assay
[0121] application[ 35 The advantages of constitutive activation of the GTPγS binding assay are: (a) [ 35 S] GTPγS binding is universal to all G protein-coupled receptors; (b)[ 35 S]GTPγS binds near the surface of the cell membrane, where molecules affecting intracellular cascades are less likely to be encountered. Preferably a GPCR: fusion protein is used. This test utilizes G protein-coupled receptor stimulation [ 35 S] The ability of GTPγS to bind to cell membranes expressing relevant receptors. Therefore, this assay can be used to directly screen compounds at the disclosed 5-HT receptors.
[0122] A scintillation proximity experiment was used to monitor [ 35 S] GTPγS interacts with endogenous human 5-HT expressed in, for example, COS cells 2C Receptor cell membrane binding. Briefly, a preferred experimental procedure is to prepare the test substance in 20 mM HEPES, pH 7.4, containing 0.3 nM [ 35 S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com