Perylene pigment compositions
A technology of perylene pigments and compositions, applied in the directions of anthracene dyes, organic dyes, organic chemistry, etc., can solve problems such as no description of perylene diimides and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0089] Examples 1-5 describe the preparation of perylene pigment compositions and the analysis of the particle size characteristics of the compositions.
Embodiment 1
[0096] Embodiment 1 (comparison)
[0097] A suspension of 120 g (0.31 mol) of perylenedicarbonyltetracarboxylic dianhydride ("PTCA) in 1600 g of water was heated with stirring to 90°C and maintained at 90°C for 4 hours. The suspension was then cooled to 23°C, Then 120 g (1.55 mol) of 40% monomethylamine in water were added dropwise over a period of 15 minutes. Once the addition was complete, the reaction mixture was stirred at 25° C. for another 45 minutes, heated to 80° C., and maintained at 80° C. for 4 hours. After reaching room temperature, the pigment was collected by vacuum filtration, washed free of amine, and dried to obtain approximately 120 g of product.
[0098]The test results are shown in Table 1.
Embodiment 2
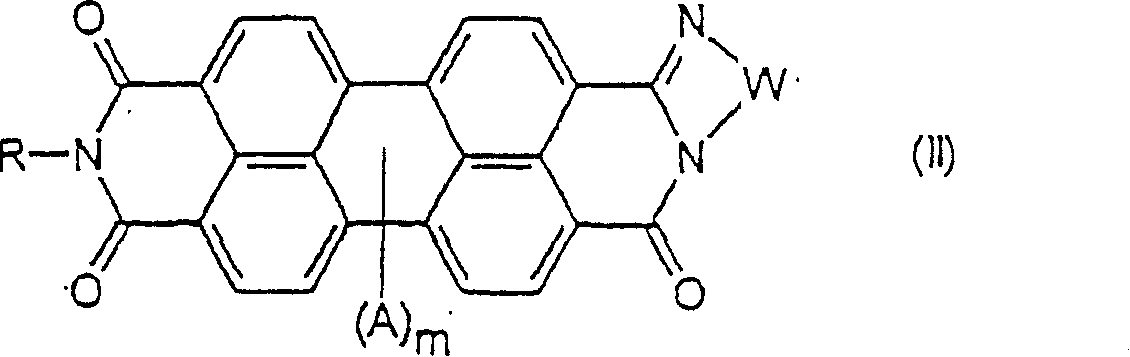
[0100] A mixture of 450 g (1.15 mol) of PTCA, 6000 g of water and 6 g (58.7 mmol) of 2,2 dimethyl-1,3-diaminopropane was stirred at 25° C. for 1 hour, heated to 90° C., and heated at 90° C. °C for 4 hours. The suspension was then cooled to 25° C., followed by the dropwise addition of 450 g (5.81 mol) of a 40% aqueous solution of monomethylamine over 15 minutes. Once the addition was complete, the reaction mixture was stirred at room temperature for an additional 45 minutes, heated to 80°C, and heated at 80°C for 4 hours. After cooling to room temperature, the pigment was collected by vacuum filtration, washed to be free of amine, and dried to obtain about 450 g of product containing about 5 mol% of an unsymmetrical perylenedicarbonylguanimide having the following general formula:
[0101]
[0102] The test results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com