Metalloporphyrin synthesizing method
A synthesis method and metalloporphyrin technology are applied in the field of metalloporphyrin synthesis, and can solve the problems of difficulty in product separation, metal ion shedding, low yield of μ-oxygen bimetalloporphyrin, etc., and achieve the effect of reducing synthesis cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
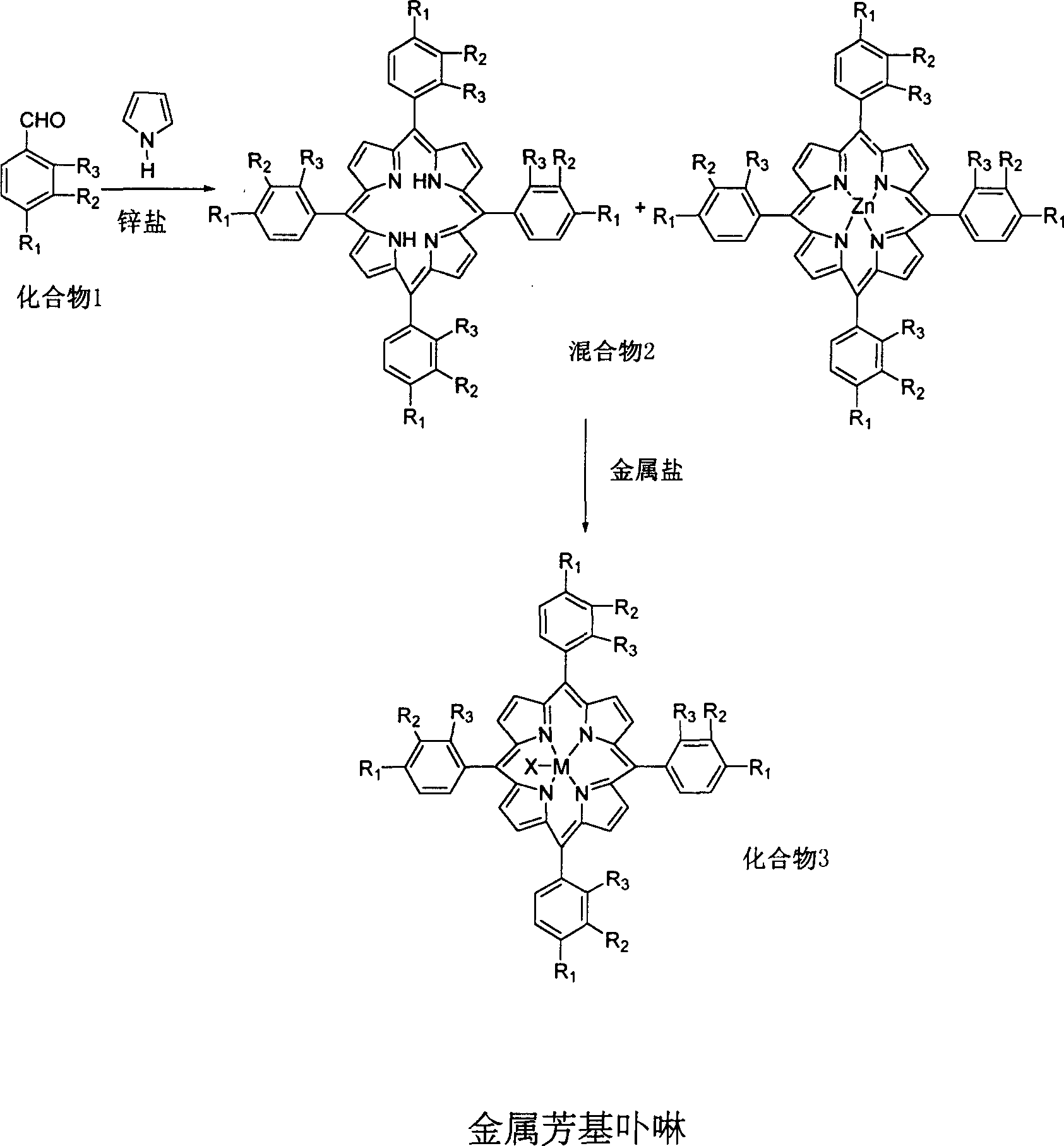
[0027] Embodiment 1: 5g compound 1, R 1 =OCH 3 , R 2 = R 3 =H, and 4g pyrrole, 5g ZnCl 2 , was added in 100ml of propionic acid, and the reactant was stirred under reflux for 0.5 hour, cooled and separated to obtain a mixture 2. The resulting mixture 2 was dissolved in refluxing xylene and 1 g Co(acac) was added 2 , reacted for 2 hours to obtain compound 3 (M=Co, R 1 =OCH 3 , R 2 = R 3 =H). The conversion of compound 1 was 30% and the conversion of mixture 2 was 95%.
Embodiment 2
[0028] Embodiment 2: with 5g compound 1, R 1 =OH, R 2 = R 3 =CH 3 , and 4g pyrrole, 5g ZnCl 2 , was added to 120ml of acetic acid, and the reactant was stirred under reflux for 1 hour, cooled and separated to obtain a mixture 2. The resulting mixture 2 was dissolved in refluxing chloroform / DMF and 1 g Cu(OAc) was added 2 , reacted for 2 hours to obtain compound 3 (M=Cu, R 1 =OCH 3 , R 2 = R 3 =H). The conversion of compound 1 was 35% and the conversion of mixture 2 was 94%.
Embodiment 3
[0029] Embodiment 3: 5g compound 1, R 1 =N(CH 3 ) 2 , R 2 = Cl, R 3 =CH 3 , and 4g pyrrole, 5gZn(OAc) 2 , was added to 120ml of acetic acid, and the reactant was stirred under reflux for 1 hour, cooled and separated to obtain a mixture 2. The resulting mixture 2 was dissolved in refluxing chloroform / DMF and 1 g NiCl was added 2 , reacted for 1.5 hours to obtain compound 3 (M=Ni, R 1 =N(CH 3 ) 2 , R 2 = Cl, R 3 =H). The conversion of compound 1 was 32% and the conversion of mixture 2 was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com