Continuous hydrogenation method of 2-nitropyridine derivative and application thereof
A technology for nitropyridine and derivatives, which is applied in the field of continuous hydrogenation of 2-nitropyridine derivatives (II), can solve the problems of long hydrogenation reaction time, high operation requirements, and difficulty in controlling spontaneous combustion of catalysts, so as to reduce impurities The risk of generation, the effect of increasing the reaction efficiency, reducing the risk of spontaneous combustion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
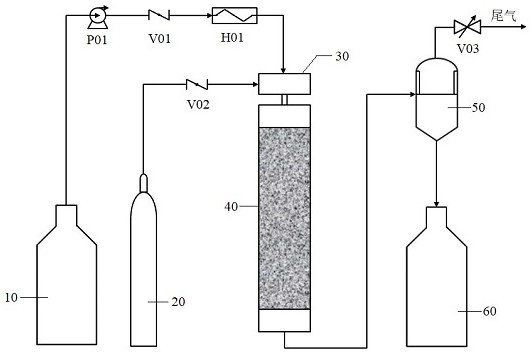
[0045] The reaction column was packed with 5% Pt / C spherical catalyst, wherein the catalyst packing mass was 2.5 g, the packing height was 31 mm, the packing diameter was 4.6 mm, and the catalyst particle size was 0.5 mm, which was used as the fixed bed reactor 40.
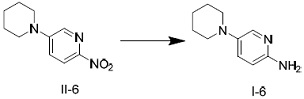
[0046] Dissolve the raw material II-1 in methanol, prepare a solution with a mass concentration of 10%, and place it in the raw material storage tank 10; ℃, mixed with hydrogen in the T-type mixer 30 to obtain a gas-liquid mixture, and the gas-liquid ratio of the gas-liquid mixture is 50:1; the above-mentioned gas-liquid mixture is transported to the fixed-bed reactor 40 for catalytic hydrogenation. Product 1-1. Among them, the reaction temperature was 30-40 °C, the reaction pressure was 1.0 MPa, and the volumetric space velocity was 6 h -1 , run for 48 h. After being separated by the gas-liquid separation device 50, the conversion rate of the raw material is 99.6%, the product purity is 99.2%, and t...
Embodiment 2
[0049]
[0050] The reaction column was packed with 5% Pt / C spherical catalyst, wherein the catalyst packing mass was 2.5 g, the packing height was 31 mm, the packing diameter was 4.6 mm, and the catalyst particle size was 0.5 mm, which was used as the fixed bed reactor 40.
[0051] Dissolve the raw material II-2 in ethanol, prepare a solution with a mass concentration of 10%, and place it in the raw material storage tank 10; ℃, mixed with hydrogen in the T-type mixer 30 to obtain a gas-liquid mixture, and the gas-liquid ratio of the gas-liquid mixture is 40:1; the above-mentioned gas-liquid mixture is transported to the fixed-bed reactor 40 for catalytic hydrogenation. Product 1-2. Among them, the reaction temperature was 40-50 °C, the reaction pressure was 2.0 MPa, and the volumetric space velocity was 6 h -1 , run for 48 h. After being separated by the gas-liquid separation device 50, the conversion rate of the raw material is 99.6%, and the product purity is 99.1%. T...
Embodiment 3
[0053]
[0054] The reaction column was packed with 5% Pt / C spherical catalyst, wherein the catalyst packing mass was 2.5 g, the packing height was 31 mm, the packing diameter was 4.6 mm, and the catalyst particle size was 0.5 mm, which was used as the fixed bed reactor 40.
[0055] The raw material II-3 was dissolved in ethyl acetate, prepared into a solution with a mass concentration of 8%, and placed in the raw material storage tank 10; the raw material solution was preheated to 40 through the plunger pump P01, the check valve V01 and the heat exchanger H01 ~50 ℃, mix with hydrogen in the T-type mixer 30 to obtain a gas-liquid mixture, and the gas-liquid ratio of the gas-liquid mixture is 30:1; the above-mentioned gas-liquid mixture is transported to the fixed-bed reactor 40 for catalytic hydrogenation reaction , the product I-3 was obtained. Among them, the reaction temperature was 40-50 °C, the reaction pressure was 3.0 MPa, and the volumetric space velocity was 6 h -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com