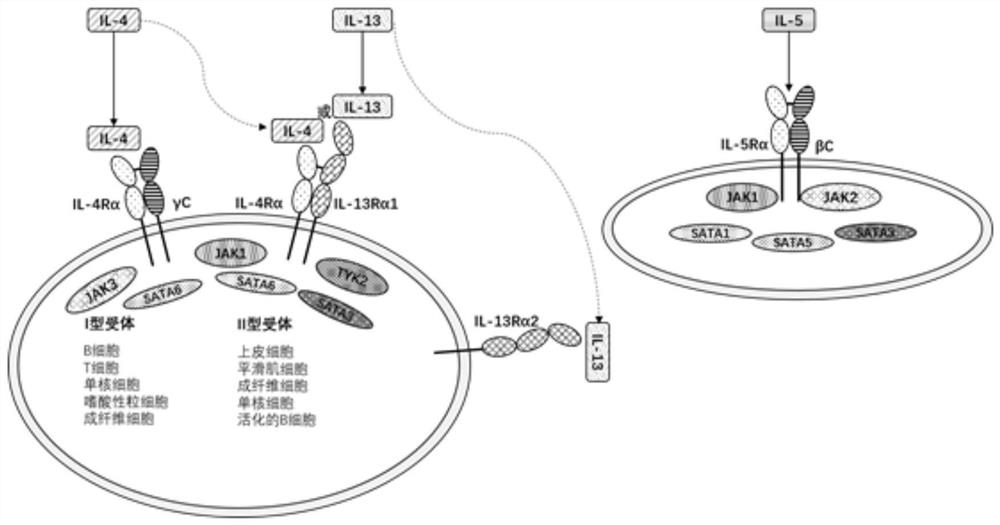
Humanized anti-IL-4R alpha single-domain antibody and application thereof
A single-domain antibody, humanized technology, applied in the direction of antibody, application, anti-animal/human immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Humanization
[0055] Humanized transformation was carried out on the basis of the anti-IL-4Rα single domain antibody of SEQ ID NO.22 (named 4E9-V0).
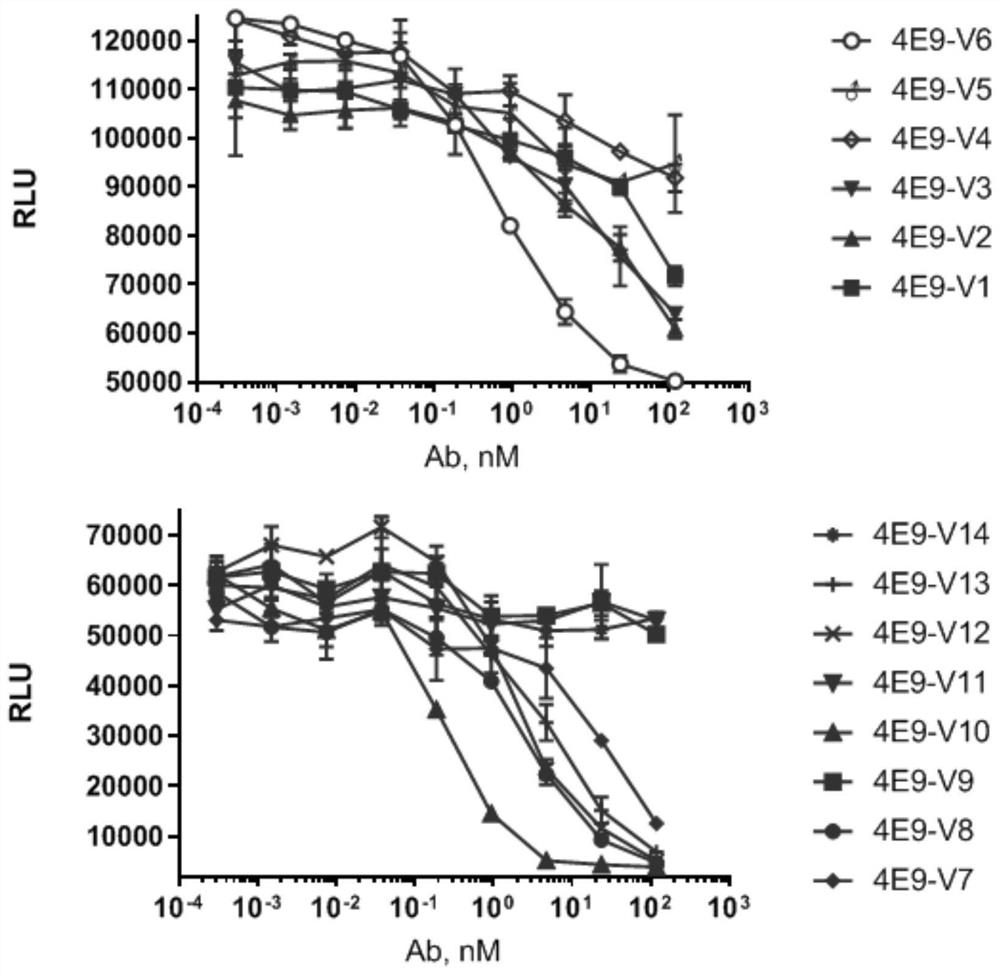
[0056] The sequences of the transformed humanized single domain antibodies (respectively named 4E9 V1-V14) are shown in Table 1, and the alignment results of the partially humanized antibody sequences are shown in figure 2 shown.
[0057] Table 1 Sequence identifiers (SEQ ID NO.) corresponding to each region of each humanized single domain antibody
[0058] FR1 CDR1 FR2 CDR2 FR3 CDR3 FR4 4E9-V0 1 3 4 12 13 18 20 4E9-V1 2 3 5 12 14 18 21 4E9-V2 2 3 6 12 14 18 21 4E9-V3 2 3 6 12 15 18 21 4E9-V4 1 3 4 12 13 19 20 4E9-V5 2 3 5 12 14 19 21 4E9-V6 2 3 7 12 15 18 21 4E9-V7 2 3 8 12 16 18 21 4E9-V8 2 3 9 12 16 18 21 4E9-V9 2 3 10 12 15 18 21 4E9-V10 2 3 11 12 15 18 21 ...
Embodiment 2
[0060] Detection of TF1 cell proliferation induced by humanized single domain antibody neutralizing IL-4 or IL-13
[0061] (1) Place 10,000 TF-1 cells passaged 3-4 times after recovery into 96-well plates;
[0062] (2) Different Tabs and humanized single domain antibodies in Table 1 were prepared as a 10 μg / mL solution, and diluted 5-fold;
[0063] (3) The Tab antibody (the reference application number is CN202010576200.7, the invention name is anti-IL-4Rα single domain antibody and the method obtained by the Chinese invention patent of application and medicine), humanized single domain antibody and EC80 concentration of IL-4 or IL13 (reference application number is CN202010576200.7, the invention name is anti-IL-4Rα single domain antibody and the method obtained by the Chinese invention patent of application and medicine) is mixed at 1:1 to make a mixed solution ;
[0064] (4) Add the mixed solution in the previous step to the cell culture well by an equal volume of the cel...
Embodiment 3
[0073] Detection of thermal stability of humanized single domain antibody by differential scanning method
[0074] experimental method:
[0075] (1) Add 45 μL of 0.1 mg / mL humanized single domain antibody solution above to 8-tube or 96-well PCR plate, then add 5 μL of 100×Sypro orange dye, and the final concentration of the dye is 5×; Each sample was repeated 3 times, and 1×PBS was used as a blank.
[0076] (2) Experiment type Melt curve, reporter group: ROX, quencher group: None; heating program: 25°C for 5 minutes, scanning range 25-95°C, heating rate 1% (about 1°C / min).
[0077] (3) Take the temperature corresponding to the maximum value of the first derivative of the melting curve as the corresponding denaturation temperature (Tm value) of the protein.
[0078] The experimental results are shown in Table 4.
[0079] Table 4
[0080]
[0081]
[0082] The results show that among all humanized single domain antibodies, 4E9-V13 has the highest thermal stability, 4E9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com