Application of chlorpromazine hydrochloride in treatment of endometrial cancer
A kind of endometrial cancer, chlorpromazine hydrochloride technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
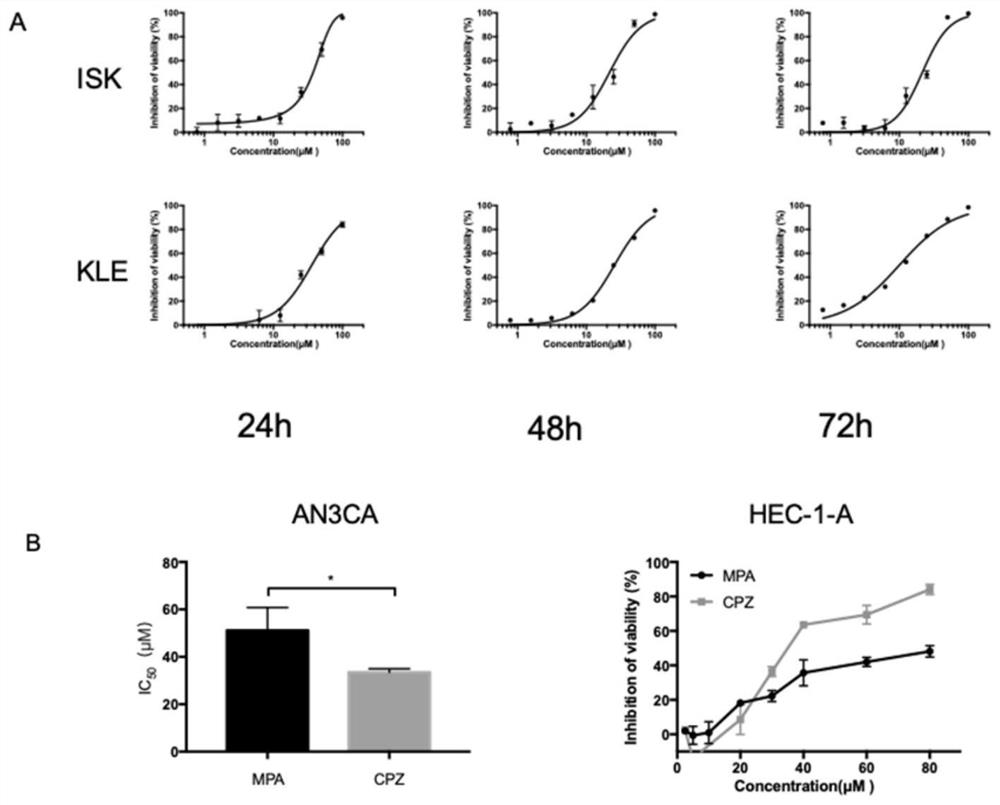
[0085] Example 1 Chlorpromazine hydrochloride against endometrial cancer cells ISK (ISK, type I, progesterone-sensitive), KLE cells (type II, progesterone-resistant), HEC-1A (type II) and AN3CA (type I, Proliferation inhibitory effect of FGFR2 mutation)
[0086] 1.1 Experimental materials and methods
[0087] Endometrial cancer cells ISK, KLE, HEC-1A and AN3CA were purchased from American tissue culture bank ATCC; phosphate buffered saline (PBS) and DMEM / F12 medium were purchased from Hyclone; serum (FBS) was purchased from Gibco Company; Pancreatin and CCK8 were purchased from Biyuntian Biotechnology Co., Ltd.; Chlorpromazine Hydrochloride (CPZ) and Medroxyprogesterone Acetate (MPA) were obtained from the laboratory old drug storehouse. The specific experimental methods are as follows:
[0088] 1) Take the cells in the logarithmic growth phase, pour out the culture medium in the petri dish, and add 2 mL of PBS to wash the cells twice.
[0089] 2) Add 1 mL of trypsin and di...
Embodiment 2
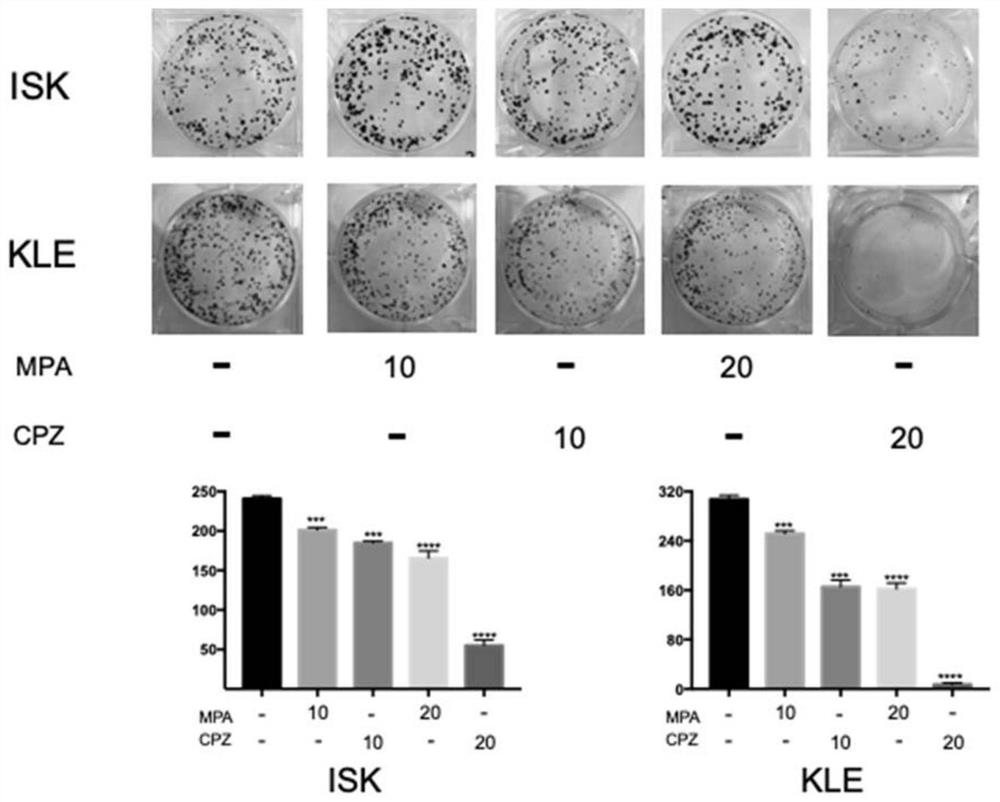
[0099] Example 2 The effect of chlorpromazine hydrochloride and MPA on ISK and KLE cell clone formation
[0100] 2.1 Experimental materials and methods
[0101] Crystal violet was purchased from Biyuntian Biotechnology Co., Ltd.; methanol was a commonly used laboratory reagent and was purchased commercially without any treatment. The sources of other experimental materials are the same as those in Example 1. The specific experimental methods are as follows:
[0102] 1) Take the cells in the logarithmic growth phase, digest them with trypsin to prepare a single-cell suspension, dilute them in a gradient manner, and inoculate them into a 6-well plate at a density of about 800 cells per well, and allow them to adhere overnight.
[0103] 2) Five experimental groups were set up, namely control group; MPA 10 μM group; MPA 20 μM group; CPZ 5 μM group; CPZ 10 μM group; CPZ 20 μM group. The administration group was cultured with 1 mL of DME / F12 medium containing different concentrat...
Embodiment 3
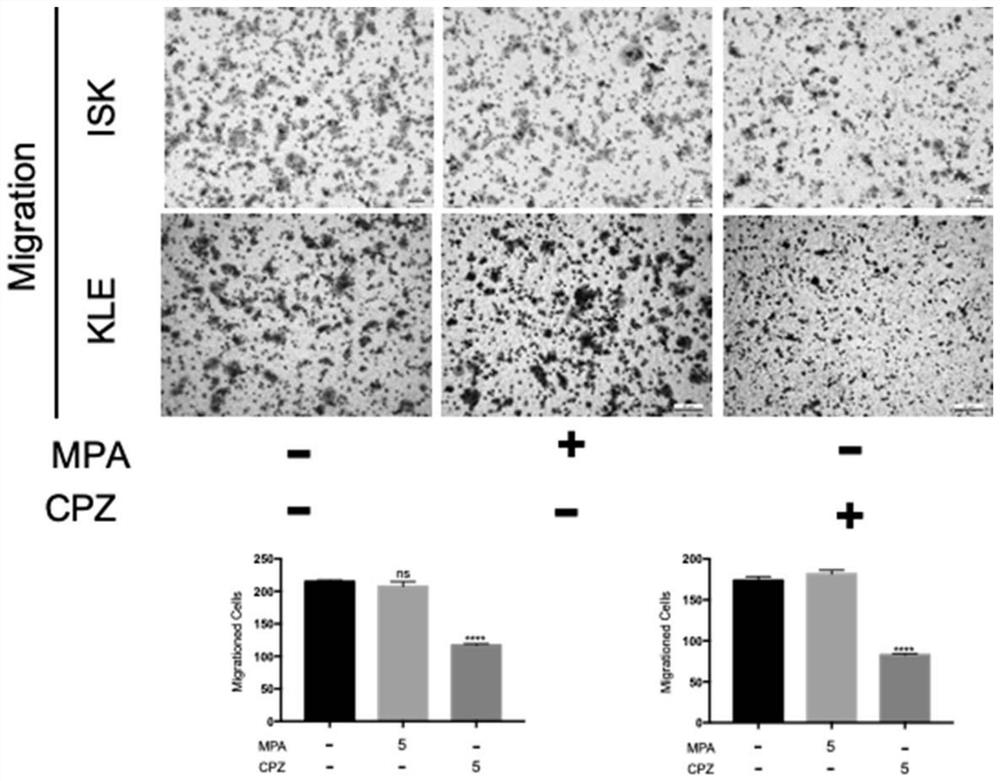
[0112] Example 3 The effect of chlorpromazine hydrochloride and MPA on the migration ability of ISK and KLE cells
[0113] 3.1 Experimental materials and methods
[0114] Paraformaldehyde is a reagent commonly used in the laboratory, purchased commercially, without any treatment, and the sources of all other experimental materials are the same as in Example 1, and the concrete experimental method is as follows:
[0115] 1) Take 100 μL of serum-free ISK / KLE cell suspension (about 200,000 cells) and add it to the upper chamber of the Transwell chamber. The medium used for the cell suspension was serum-free DME / F12 medium.
[0116] 2) Add 600 μL of DME / F12 medium containing 20% FBS to the lower chamber, taking care to avoid the generation of air bubbles.
[0117] 3) Set up 3 experimental groups, namely control group; MPA 5μM group; CPZ 5μM group. 100 μL of serum-free DME / F12 medium containing different drugs was added to the upper chamber of the administration group. 100 μL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com