Novel immunochromatography detection device
An immunochromatographic detection and detection device technology, applied in the field of biological detection, can solve the problems of not the most simplified operation steps, operation errors, increased virus transmission, etc., and achieve the effect of reducing utilization, convenient use, and reducing the risk of transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 The novel immunochromatographic detection device provided by the present invention
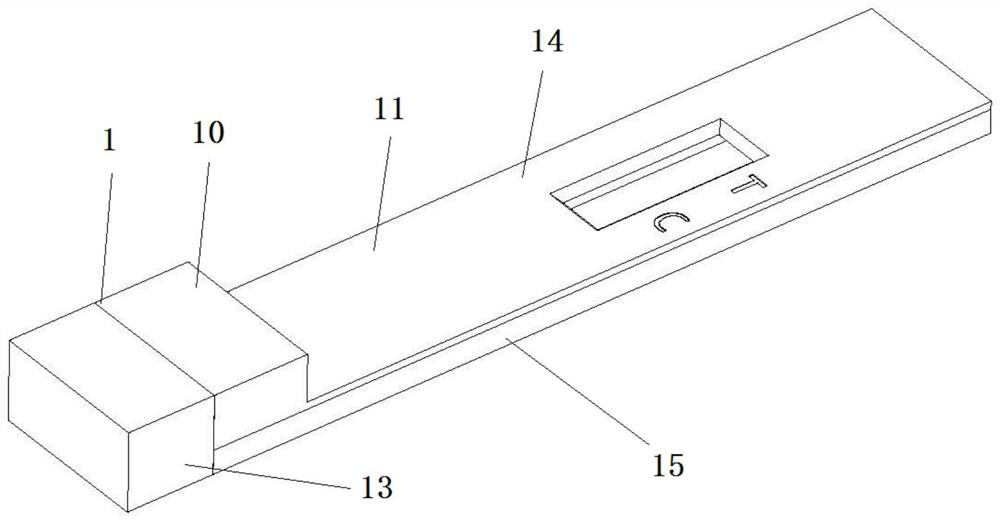
[0064] The novel immunochromatographic detection device provided in this embodiment is as Figure 1-3 shown, where figure 1 It is a schematic diagram of the structure of the new immunochromatographic detection device, figure 2 It is a split diagram of each layer structure of the new immunochromatography detection device, image 3 It is a structural disassembly diagram of the sample collection part of the new immunochromatography detection device.
[0065] Such as figure 1 , the present invention provides a novel immunochromatographic detection device, the detection device includes a sample collection site 1 and a test strip 2, the test strip 2 includes a sample pretreatment pad 3, and the sample collection site 1 collects The sample can be transferred to the sample pretreatment pad 3, and the sample pretreatment pad 3 has a dry and fixed sample pretreatment liquid on it. ...
Embodiment 2
[0076] Example 2 Preparation of an immunochromatographic detection device (colloidal gold method) for detecting novel coronavirus
[0077] The preparation steps of the immunochromatographic detection test device (colloidal gold method) for detecting novel coronavirus SARS-CoV-2 provided by this embodiment include:
[0078] Step 1: Prepare the sample pretreatment pad
[0079] 1) Prepare phosphate buffer and adjust the pH to 7.5.
[0080] 2) Take a certain mass fraction of the above phosphate buffer: 0.01mol / L, a certain mass fraction of casein: 3%, a certain mass fraction of Triton X-100: 1%, a certain mass fraction of proclin 300: 0.01% configuration into a pretreatment solution.
[0081] 3) Evenly disperse the pretreatment solution on the glass fiber, dry overnight in a 37°C drying oven, take it out and seal it in an aluminum foil bag for later use.
[0082] Step 2: Sample Pad Processing
[0083] 1) Configure boric acid buffer and adjust the pH to 7.5.
[0084] 2) Take a...
Embodiment 3
[0103] Example 3: Preparation of another immunochromatographic detection device (colloidal gold method) for detecting novel coronavirus
[0104] Same as Example 2, the difference is to use stearic acid monoethanolamide instead of TritonX-100 in the preparation of the pretreatment pad, and all the other ingredients are the same as other contents in Example 2, and according to the mass fraction of stearic acid monoethanolamide Different, divided into three recipes for the preparation of pre-treatment pads.
[0105] Formula 1: 0.01 mol / L phosphate buffer, 3% casein, 0.5% stearic acid monoethanolamide, and 0.01% proclin 300.
[0106] Formula 2: 0.01 mol / L phosphate buffer, 3% casein by mass fraction, 1% stearic acid monoethanolamide, 0.01% proclin 300 by mass fraction.
[0107] Formula 3: 0.01 mol / L phosphate buffer, 3% casein by mass fraction, 1.5% stearic acid monoethanolamide, 0.01% proclin 300 by mass fraction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com