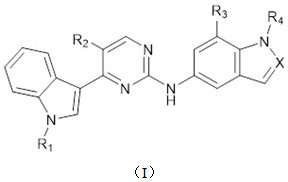
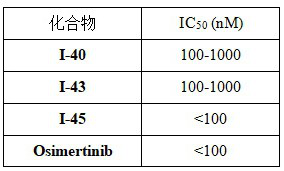
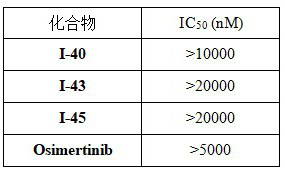
2-amino-4-indolyl pyrimidine compound as well as preparation method and application thereof
A compound, amino technology, applied in the field of chemical medicine, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: N-(1-(2-(dimethylamino)ethyl)-5-((4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl)amino) -1H-indazol-7-yl)acrylamide (I-1)
[0076]
[0077] The synthetic route is as follows:
[0078]
[0079] Step 1. Preparation of 5,7-dinitro-1H-indazole (2):
[0080] Under ice bath, 5-nitro-1H-indazole (1) (16.32g, 100mmol) was dissolved in sulfuric acid solution, and concentrated nitric acid (40ml, 400mmol) was added dropwise under ice bath. After the reaction was completed, the reaction solution was Slowly pour into ice water, stir well, and filter with suction to obtain 20.61 g of white filter cake of 5,7-dinitro-1H-indazole (2), with a yield of 99%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 14.57(s, 1H), 9.30 (d, J = 2.0 Hz, 1H), 8.95 (d, J = 2.0 Hz, 1H), 8.70 (s, 1H).
[0081] Step 2. Preparation of 2-(5,7-dinitro-1H-indazol-1-yl)-N,N-dimethyl-1-ethylamine (3):
[0082]Dissolve 5,7-dinitro-1H-indazole (2) (10.41g, 50mmol) in THF, add sodium hydrogen (1.44g, 60mmol) un...
Embodiment 2
[0095] Example 2: (E)-N-(1-(2-(Dimethylamino)ethyl)-5-((4-(1-methyl-1H-indol-3-yl)pyrimidine-2- Base)amino)-1H-indazol-7-yl)-2-butenamide (I-2)
[0096]
[0097] Referring to the synthetic method of (I-1), the yield was 66%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 11.26 (s, 1H),8.38 (d, J = 8.0 Hz, 1H), 8.35 (d, J = 5.3 Hz, 1H), 8.19 – 7.99 (m, 3H), 7.93(s, 1H), 7.37 (d, J = 8.2 Hz, 1H), 7.33 – 7.27 (m, 1H), 7.24 – 7.21 (m, 1H),7.13 – 7.07 (m, 1H), 7.05 (s, 1H), 6.06 (d, J = 15.2 Hz, 1H), 4.69 (dd, J =5.9, 3.6 Hz, 2H), 3.89 (s, 3H), 2.92 (s, 2H), 2.24 (s, 6H), 1.95 (d, J = 6.9Hz, 3H).
Embodiment 3
[0098] Example 3: N-(1-(2-(dimethylamino)ethyl)-5-((4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl)amino) -1H-indazol-7-yl)-3-methyl-2-butenamide (I-3)
[0099]
[0100] Referring to the synthetic method of (I-1), the yield is 57%. 1 H NMR (400 MHz, Chloroform- d ) δ 11.18(s, 1H), 8.41 (dt, J = 7.8, 1.0 Hz, 1H), 8.35 (d, J = 5.3 Hz, 1H), 8.12 –7.91 (m, 4H), 7.39 – 7.28 (m, 2H), 7.25 – 7.18 (m, 2H), 7.05 (d, J = 5.4 Hz,1H), 5.86 – 5.80 (m, 1H), 4.71 – 4.66 (m, 2H), 3.88 (s, 3H), 2.90 (s, 2H),2.29 (d, J = 1.3 Hz, 3H), 2.24 (s, 6H), 1.94 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com