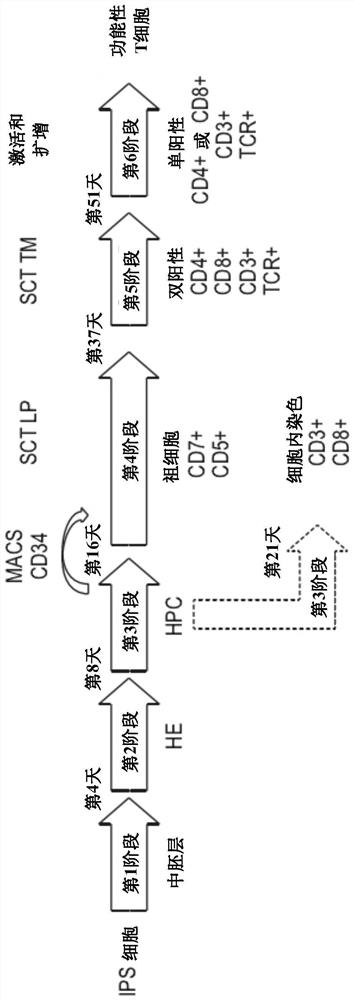
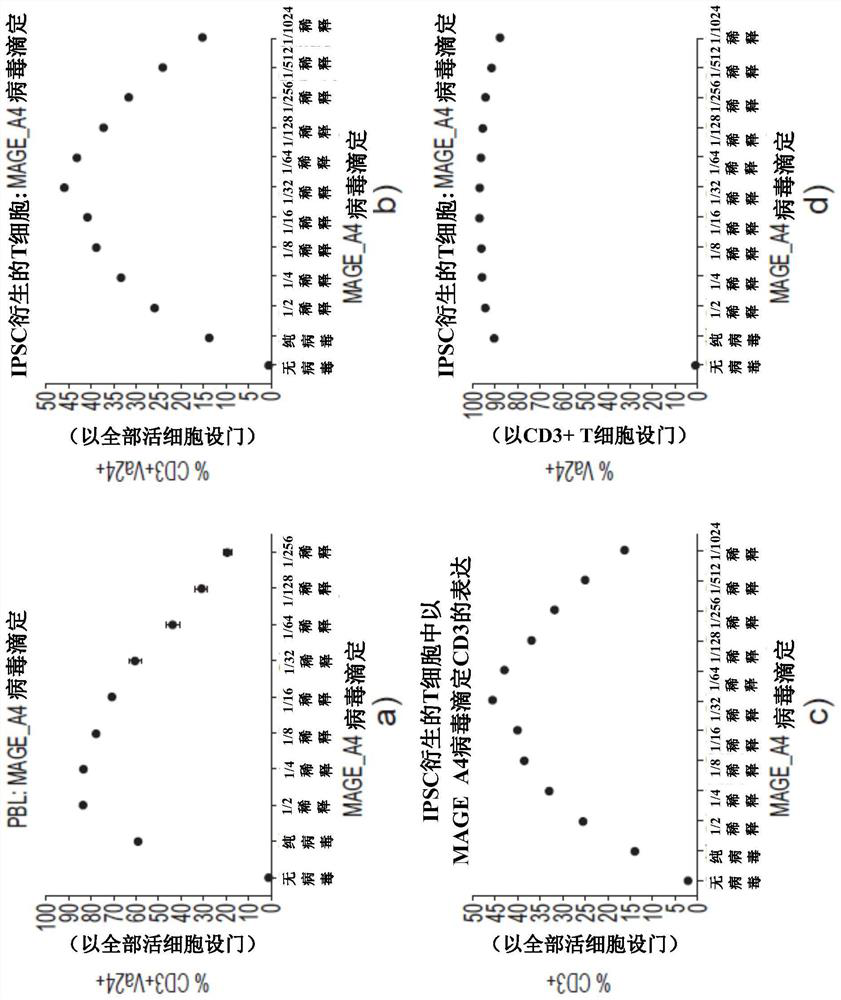
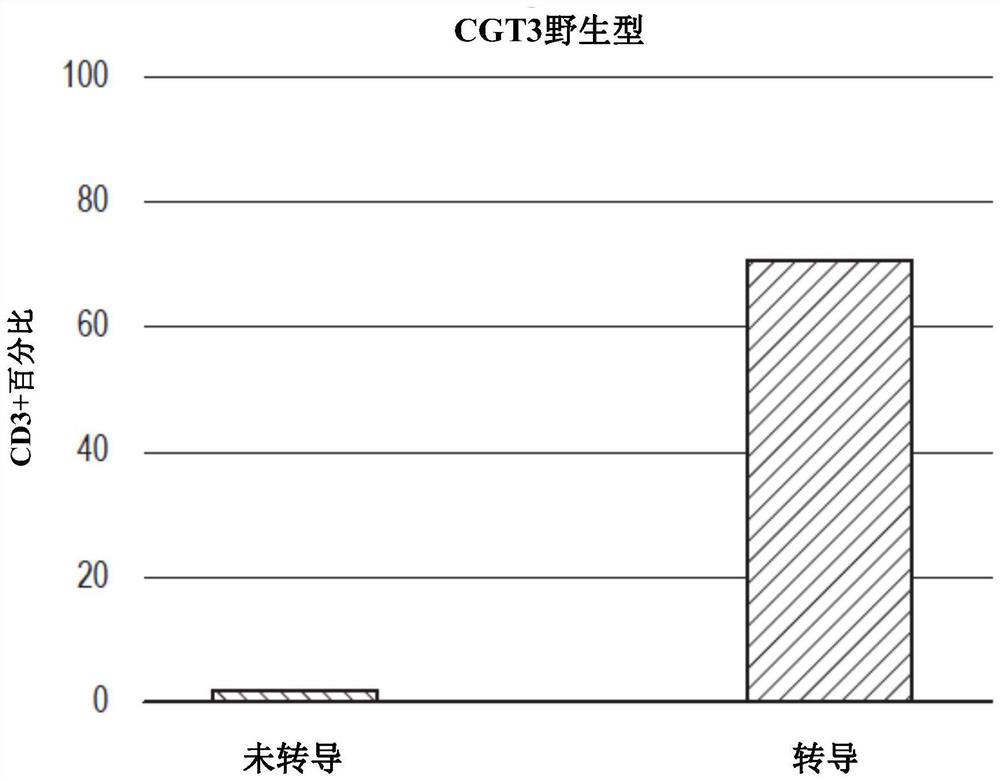
Production of T cells from RAG-inactivated iPSC
A technology of cells and cell groups, applied in the field of progenitor cells, which can solve problems such as lack and limitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0208] method
[0209] hiPSC culture
[0210] iPSCs are routinely cultured in mTeSR1(SCT) on Matrigel (BD brand, Corning "Corning") using tissue culture plasticware in 5% CO 2 , 5% O 2 and 37°C. HiPSCs were obtained according to the instructions using the EasyPassage tool (Invitrogen), and seeded in a medium with 10 μm Y27632 (Andy Biotechnology "R&D Systems") at a ratio of 1:6 or 1:12 in the first 48 hours of culture. For differentiation, hiPSCs were passaged in low-density culture onto matrigel or vitronectin using a split ratio of 1:48 or 1:98. 24 hours after inoculation, the inoculation density was approximately 1 colony per field of view when observed under a microscope with a magnification of ×4. hiPSCs were cultured in mTeSR2 or E8flex (SCT) for approximately 4-5 days, depending on the cell culture substrate used, until colonies became dense and distinct cells were no longer visible.
[0211] T cell differentiation from pluripotent stem cells
[0212] The hi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com