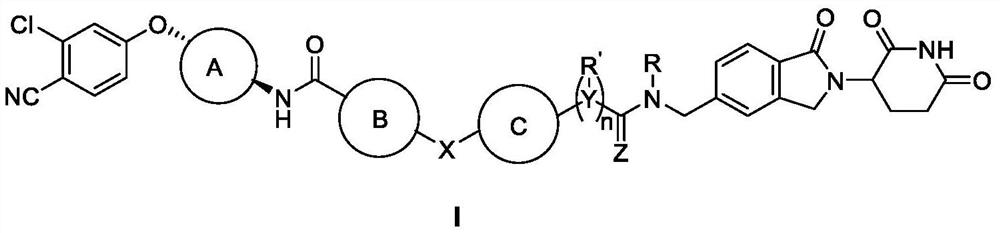
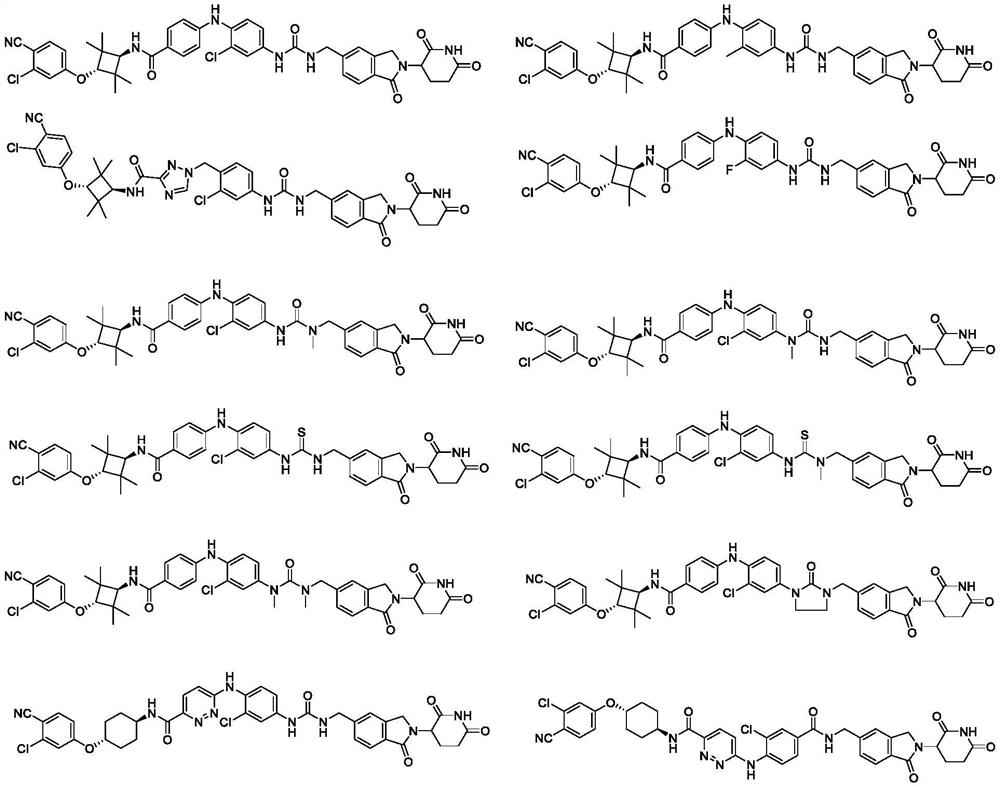Bifunctional molecule as well as preparation method and application thereof
A technology of heteroatoms and compounds, applied in the field of drug compounding, can solve the problem of single structure of androgen receptor inhibitors, and achieve the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Embodiment 1: the synthesis of intermediate 1
[0154]
[0155] Dissolve tert-butyl ((1r,3r)-3-hydroxy-2,2,4,4-tetramethylcyclobutane)carbamate (3.1g, 12.86mmol, 1eq) in anhydrous THF at 0°C Sodium hydrogen (617mg, 15.43mmol, 60%, 1.2eq) was added under conditions, and after stirring under nitrogen for 30min, 2-chloro-5-fluorobenzonitrile (2g, 12.86mmol, 1eq) was added and stirred at room temperature for 4h. After the reaction was detected by TLC, water was added to quench the reaction, THF was removed under reduced pressure, diluted with ethyl acetate (100 mL), washed with water (50 mL), washed with saturated brine (50 mL), dried over anhydrous sodium sulfate and concentrated, the residue 3.5 g of the product was obtained by column chromatography (PE / EA 10:1). Dissolve the above product in 50 mL of dioxane, drop into HCl in dioxane solution (4M, 10 mL), stir overnight at room temperature, filter with suction, wash the filter cake with dioxane and ether, and dry to ...
Embodiment 2
[0156] Embodiment 2: the synthesis of intermediate 2
[0157]
[0158] The above product (compound 1-4) (50mg, 0.13mmol, 1eq) was dissolved in DMSO, and 4-fluoronitrobenzene (21mg, 0.15mmol, 1.2eq) and DIEA (31μL, 0.19mmol, 1.5eq) were added , Stir overnight at 120 degrees. After the reaction was detected by TLC, it was diluted with ethyl acetate (20 mL), washed with water (20 mL), washed with saturated brine (20 mL), dried over anhydrous sodium sulfate and concentrated. The residue was subjected to column chromatography to obtain 28 mg of the product. Dissolve the above product in THF, add palladium carbon, stir overnight at room temperature under hydrogen, filter with suction, and concentrate the filtrate to obtain 25 mg of the product, ESI MS m / z 490[M+H] + .
Embodiment 3
[0159] Embodiment 3: the synthesis of intermediate 3
[0160]
[0161] The above product (compound 1-4) (50mg, 0.13mmol, 1eq) was dissolved in DMSO, and 3,4-difluoronitrobenzene (24mg, 0.15mmol, 1.2eq) and DIEA (31μL, 0.19mmol, 1.5eq), stirred overnight at 120 degrees. After the reaction was detected by TLC, it was diluted with ethyl acetate (20 mL), washed with water (20 mL), washed with saturated brine (20 mL), dried over anhydrous sodium sulfate and concentrated. The residue was subjected to column chromatography to obtain 36 mg of the product. Dissolve the above product in THF, add palladium carbon, stir overnight at room temperature under hydrogen, filter with suction, and concentrate the filtrate to obtain 35 mg of the product, ESI MS m / z 508 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com