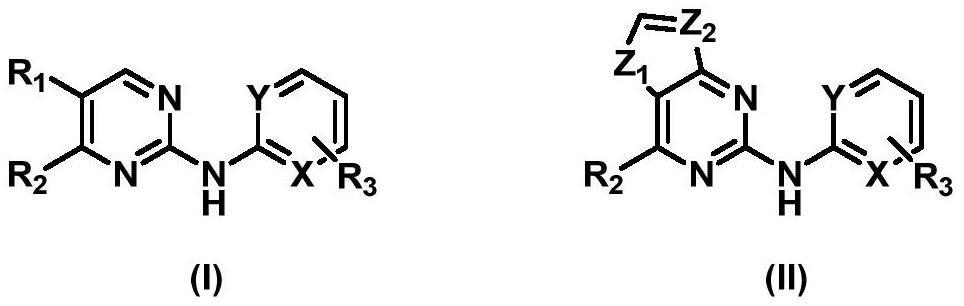
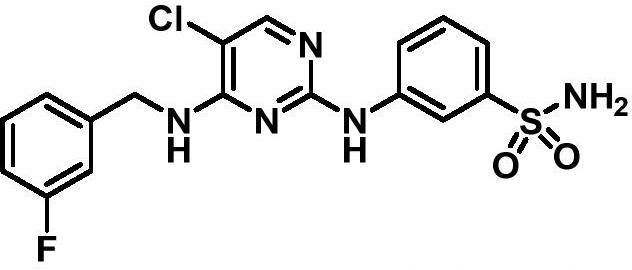
Aminopyrimidine derivative as well as preparation method and application thereof
A technology of aminopyrimidine and derivatives, applied in TRK inhibitors, aminopyrimidine derivatives, preparation of therapeutic agents, and its stereoisomers, can solve the problems of no longer effective, loss of TRK protein ectodomain, etc., and achieve good TRK inhibition active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] The preparation route of embodiment 1 is as follows:
[0147]
[0148] Concrete synthetic steps are as follows:
[0149] Synthesis of 2,5-dichloro-N-(3-fluorobenzyl)pyrimidin-4-amine (2)
[0150] Dissolve 2,4,5-trichloropyrimidine (1.00g, 5.50mmol) in 15mL of absolute ethanol, add N,N-diisopropylethylamine (1.00mL, 6.05mmol), stir at room temperature for 10min, and add to the reaction solution Add a solution of 3-fluorobenzylamine (0.69mL, 6.05mmol) in absolute ethanol (15mL) dropwise, and react at room temperature. The completion of the reaction of the raw materials was monitored by TLC, the solvent was removed by spin, and purified by column chromatography to obtain a light yellow solid with a yield of 88%.
[0151] Preparation of 4-({5-chloro-4-[(3-fluorobenzyl)amino]pyrimidin-2-yl}amino)benzenesulfonamide (Example 1)
[0152] Intermediate 2 (0.10 g, 0.37 mmol) was dissolved in 3 mL of isopropanol, 4-aminobenzenesulfonamide (0.070 g, 0.41 mmol) and a catalytic ...
Embodiment 14
[0177] The preparation route of embodiment 14 is as follows:
[0178]
[0179] Preparation of 4-({5-chloro-4-[(3-fluorobenzyl)amino)pyrimidin-2-yl)aminobenzoic acid (4)
[0180] Referring to the method of Preparation Example 1, the 4-aminobenzenesulfonamide raw material in step b was replaced with p-aminobenzoic acid in equal proportions to obtain intermediate 4.
[0181] [4-({5-chloro-4-[(3-fluorobenzyl)amino]pyridin-2-yl}amino)phenyl](4-methylpiperazin-1-yl)methyl ketone (Example 14) Preparation
[0182] Intermediate 4 (0.10g, 0.27mmol), N-methylpiperazine (0.032g, 0.32mmol), EDCI (0.061g, 0.32mmol), HOBt (0.043g, 0.32mmol) and DIPEA (0.053mL, 0.32 mmol) was dissolved in 3mL DMF and reacted at room temperature. TLC monitored the complete reaction of the raw materials, and the reaction solution was poured into 30 mL of 10% potassium carbonate solution, and a white solid was precipitated. It was filtered under reduced pressure, and the filter cake was washed with water ...
Embodiment 29
[0210] Embodiment 29 adopts following route to prepare:
[0211]
[0212] Preparation of 3-({5-chloro-4-[(3-fluorobenzyl)amino]pyrimidin-2-yl}amino)-N-(piperidin-4-yl)benzamide (Example 29)
[0213] Referring to the method of Preparation Example 17, the morpholine raw material in step c was replaced by N-Boc-piperidin-4-amine in equal proportions to obtain intermediate 7. Intermediate 7 (0.050 g, 0.090 mmol) was dissolved in saturated ethyl acetate solution of hydrogen chloride and stirred at room temperature (step d). TLC monitored the completion of the reaction, filtered under reduced pressure, washed the filter cake three times with ethyl acetate, dissolved the filter cake in 20 mL of water, extracted with ethyl acetate (10 mL×3), and discarded the organic layer. The aqueous layer was adjusted to pH>10 with 10% sodium hydroxide solution under ice bath conditions, extracted with ethyl acetate (10 mL×3), washed with saturated brine (10 mL×3), and dried over anhydrous sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com