Method for preparing 5-formyl-2-furancarboxylic acid through catalytic oxidation of 5-hydroxymethylfurfural
A technology for catalytic oxidation of hydroxymethylfurfural, which is applied in chemical instruments and methods, catalytic reactions, physical/chemical process catalysts, etc., can solve the problems of high energy consumption and equipment requirements, high cost and large-scale application, and achieve selective High stability, mild reaction conditions, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (NH 4 ) 3 h 6 CoMo 6 o 24 Preparation example of
[0023] Weigh 1.0583g CoSO 4 ·7H 2 O, dissolved in 7.5 mL deionized water, added 1.5 mL 30 wt% H 2 o 2 , stirred at room temperature for 30 minutes, recorded as solution 1. At the same time, weigh 7.728g (NH 4 ) 6 Mo 7 o 24 ·6H 2 O, dissolved in 65mL deionized water, placed in an oil bath and heated to boiling, recorded as solution 2. Solution 1 was added dropwise to solution 2, and the resulting solution was refluxed for 10 minutes, then evaporated for 15 minutes, left to stand overnight, filtered, washed with water, and vacuum-dried at 60°C for 12 hours to obtain Anderson type heteropolyacid salt (NH 4 ) 3 h 6 CoMo 6 o 24 .
Embodiment 2
[0025] (NH 4 ) 4 ZnM 6 o 24 Preparation example of
[0026] Weigh 0.5g ZnSO 4 ·7H 2 O, dissolved in 29 mL of deionized water, recorded as solution 1. At the same time, weigh 2.5g (NH 4 ) 6 Mo 7 o 24 ·6H 2 O, dissolved in 40mL deionized water, placed in an oil bath and heated to boiling, recorded as solution 2. Add solution 1 dropwise to solution 2, and the obtained solution is rotary evaporated for 10 minutes, then evaporated for 15 minutes, filtered while it is hot, the filtrate is left to stand for crystallization, filtered, washed with water, and dried in vacuum at 60°C for 12 hours to obtain Anderson type heteropolyacid salt (NH 4 ) 4 ZnM 6 o 24 .
Embodiment 3
[0029] Catalytic oxidation of 5-hydroxymethylfurfural to prepare 5-formyl-2-furanoic acid comprises the following steps:
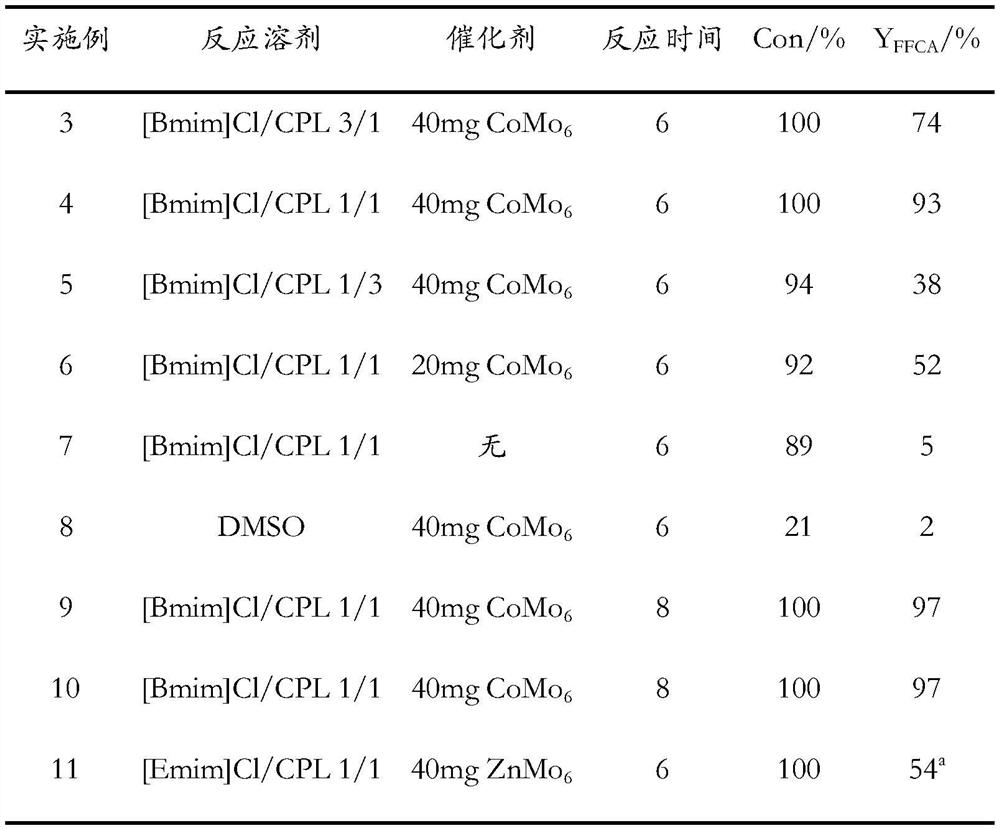
[0030] (1) Mix 1-butyl-3-methylimidazolium chloride (BmimCl) and caprolactam (CPL) in a molar ratio of 3 / 1, heat and stir at 80°C for 1 hour to obtain a homogeneous and clear liquid, That is, deep eutectic solvent (DES).
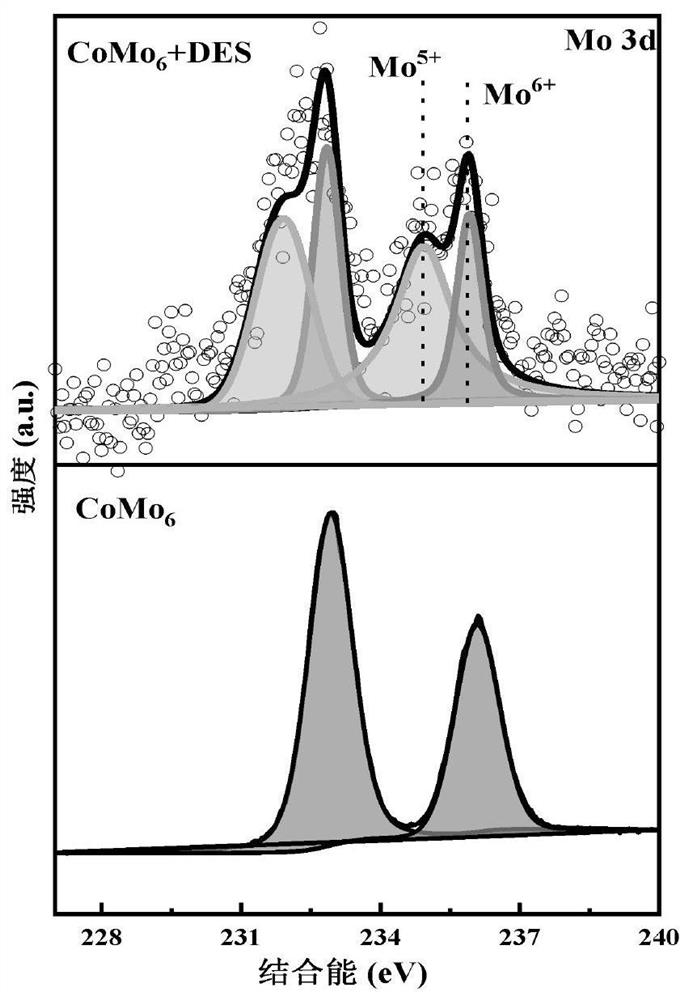
[0031] (2) Add 0.4mmol HMF to 4g of DES synthesized in step (1), then add 40mg (NH 4 ) 3 h 6 CoMo 6 o 24 Catalyst, and then continue to feed oxygen at a flow rate of 20mL / min, carry out HMF oxidation reaction at 130°C for 6h, and obtain a mixed liquid containing 5-formyl-2-furancarboxylic acid (FFCA).
[0032] (3) Dilute the mixed solution with water as a solvent, and analyze it with a high-performance liquid chromatograph, and use a standard solution to identify the product type. The results are shown in Table 1, showing that the HMF conversion rate is 100%, and the FFCA yield reaches 74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com