Anti-her2 affibody, and switchable chimeric antigen receptor using same as switch molecule
A technology of chimeric antigen receptors and affimers, which is applied in the direction of antibody medical components, anti-receptors/cell surface antigens/cell surface determinant immunoglobulins, antibodies, etc., which can solve the problem of uncontrollable chimeric antigen receptor T cells Activation and expansion, application restrictions and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1. Development of affiBody in HER2
Embodiment 1-1
[0151] Example 1-1. Screening by Panning (PANNING)
[0152] A cloning body with HER2 specific binding was screened from the affinity library (Library) using HER2-ECD-FC proteins. Further, five kinds of complexes were screened by confirming the binding clones binding to the cells expressing HER2.
[0153] The affinity library is used in phage morphological rescue by using VSCM13 auxiliary phage (Helper Phage). First, the number of library phages used to combine the antigen is 10 13More than the 4 rounds of Panning Rounds. The method used is as follows: The strategy of selectively screening the high phage of affinity, with the gradual increase in the number of times, gradually reduces the amount of antigen (10 μg, 5 μg, 2 μg, 1 μg), gradually increase the cleaning (WASH) ) Times (3 times, 5 times, 7 times, 10 times).
[0154] The binder pHAGE obtained in each wheel obtained by the infection (INFECTION) obtained by an enzyme-linked immunosorbent assay (ELISA) is used to confirm whet...
Embodiment 1-2
[0158] Example 1-2. Confirmation of the combination of parentures
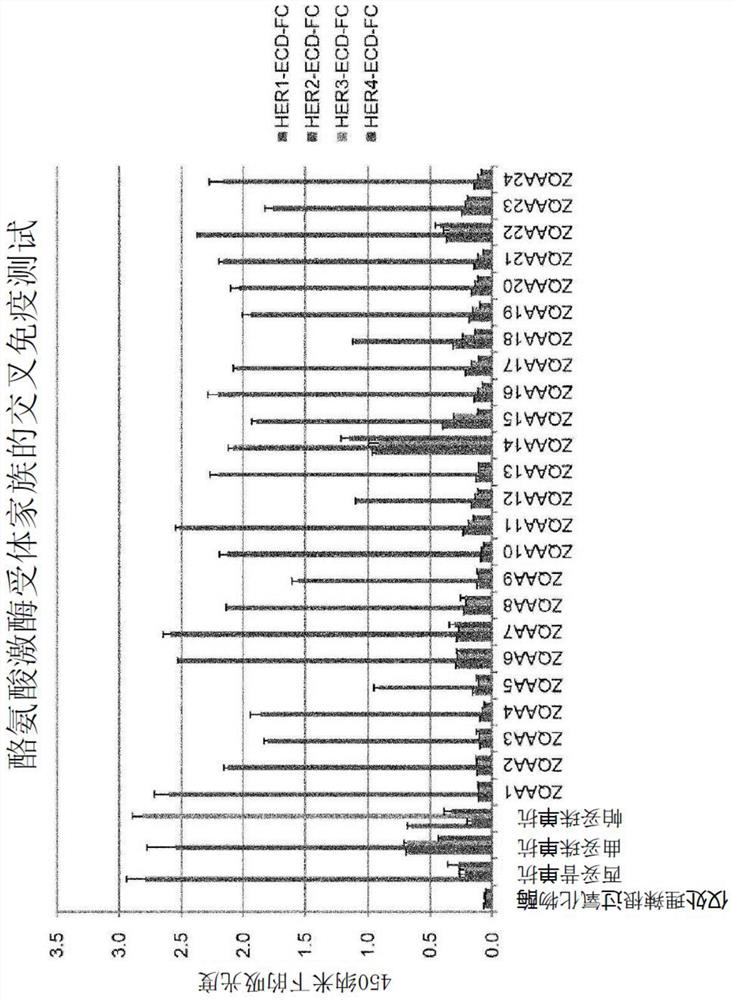
[0159] The five kinds of affinity clones (Cloning) were screened into a Fc binding form (ZB-Fc) to confirm the degree of binding to HER2 protein and the degree of binding to HER2 expression cells. The enzyme-linked immunosorbent assay was carried out by the following method: 5 ZB-Fc purified from 60 nm was diluted to 1 / 5, and the above-mentioned ZB-Fc was coated with HHER2-ECD-His. 7 points were treated on the culture plate of the protein, and the second antibody (Anti-Higg-Fc-HRP (INVITROGEN company, H10007)) was treated, 3, 3 ', 5,5'-Tetracycline is used. After the acid-linked immunosorbent assay reader is used to determine OD using an enzyme-linked immunosorbent assay reader. 450 Value, seeking EC via Graph Prism 50 value( image 3 ,Table 2).
[0160] Table 2
[0161] distinguish ZQAA1 ZQAA7 ZQAA8 ZQAA11 ZQAA22 EC 50 (nm)
3.6 68.5 1.8 6.9 N / D
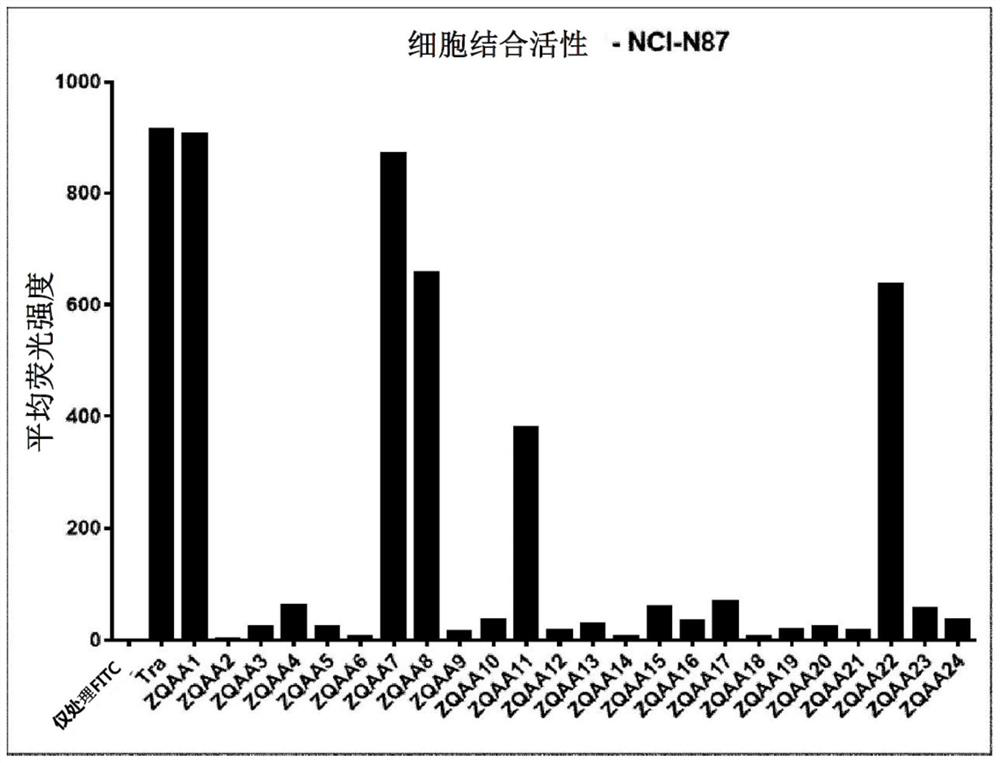
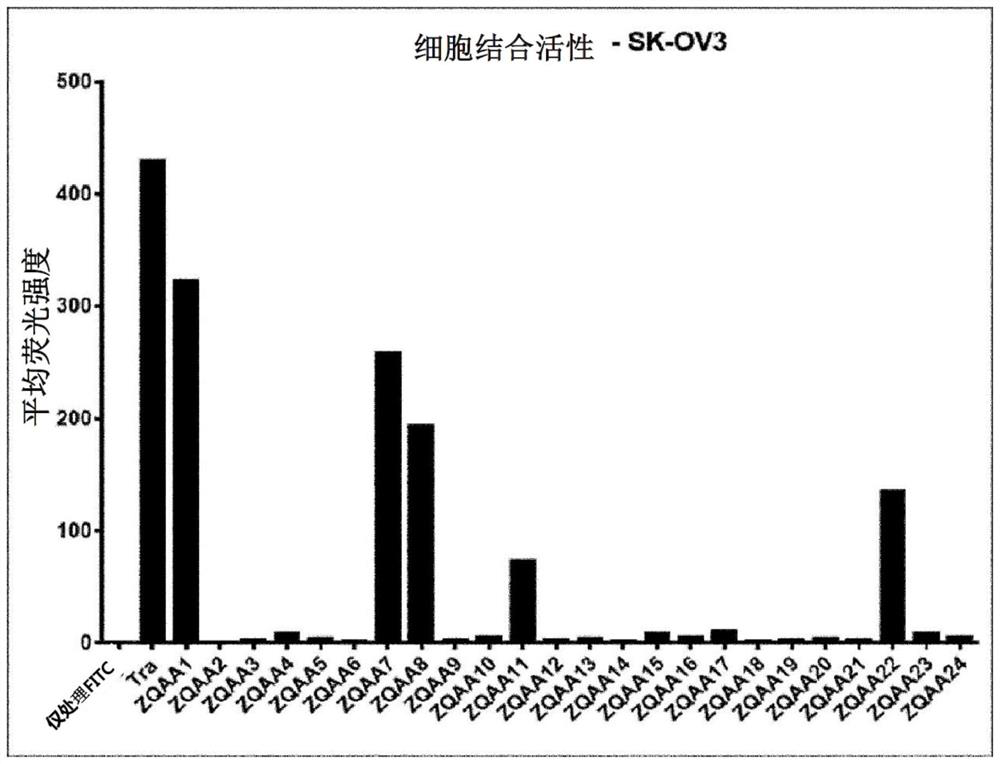
[0162] In order to confirm the degree...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com