A cyanostyrene derivative, preparation method and application thereof, polymer detection probe and fluorescence detection method
A cyanostyrene and fluorescence detection technology, applied in the direction of fluorescence/phosphorescence, measurement devices, photovoltaic power generation, etc., to achieve the effect of improving the depth of fluorescence imaging and large two-photon absorption cross-sectional area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
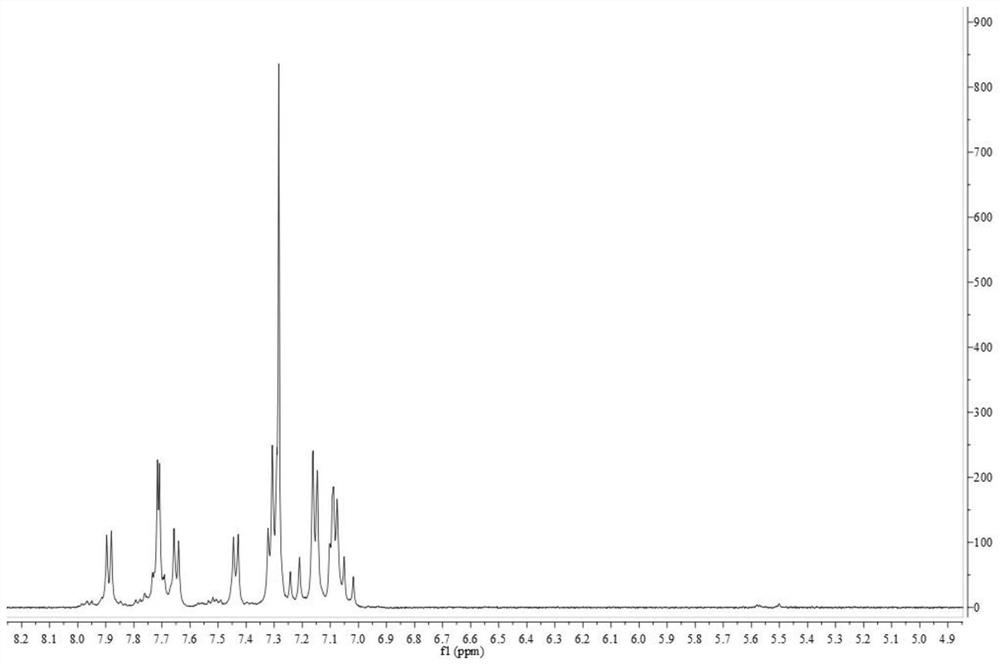
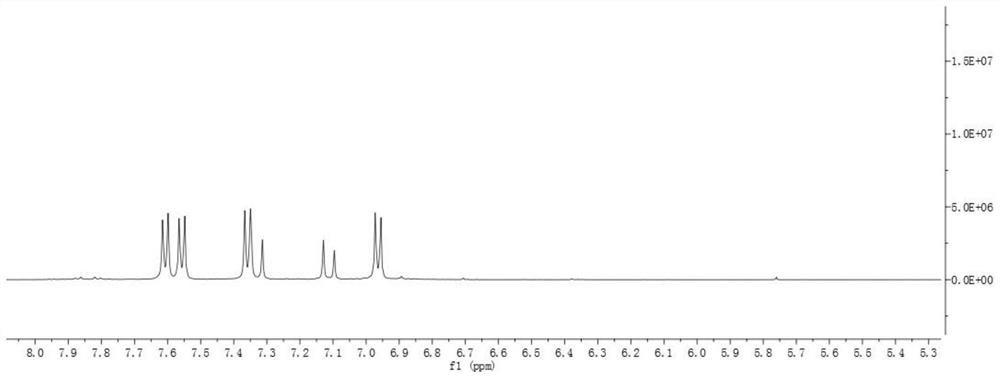
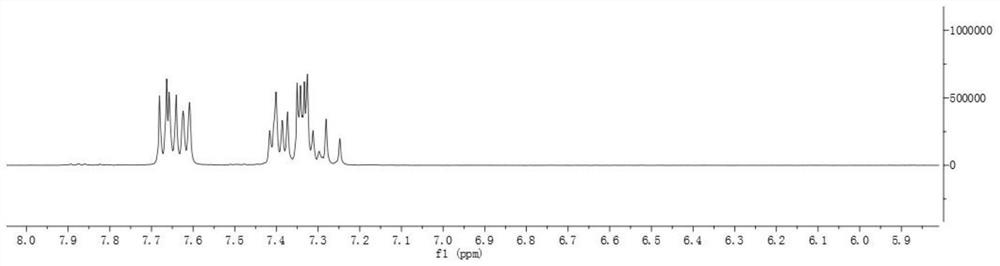
Image
Examples
preparation example Construction
[0060] The invention provides the preparation method of the cyanostyrene derivative described in the above technical scheme, comprising the following steps:
[0061] P-bromophenylacetonitrile, iodine, sodium methoxide solution and the first solvent are mixed, and bimolecular oxidative coupling reaction is carried out to obtain double bromine intermediate product;
[0062] The bis-bromo intermediate product, the compound containing electron donating group, Pd(OAc) 2 , Ag 2 CO 3 It is mixed with the second solvent to carry out a Heck reaction to obtain a cyanostyrene derivative.
[0063] The compound containing the donor group has the structure shown in formula II:
[0064] formula II; in formula II, X is
[0065] In the present invention, unless otherwise specified, the required preparation raw materials are all commercially available commodities well known to those skilled in the art.
[0066] The present invention mixes p-bromophenylacetonitrile, iodine, sodium met...
Embodiment 1
[0095] p-Bromophenylacetonitrile (5.00 g, 25.5 mmol) and iodine (6.57 g, 25.5 mmol) were dissolved in dry ether (100 mL), and sodium methoxide (2.89 g, 5.3 mmol) was dissolved in methanol solution (8.68 g, 14.61 mmol) mL), the obtained ether mixture was mixed with sodium methoxide solution, stirred for 0.5 h in a dry ice bath, heated to 0 °C, replaced by ice water in the dry ice bath, and stirred for 4 h. % hydrochloric acid to quench the reaction; after the resulting mixture was stirred for 12 hours, the resulting material was filtered, the separated solid obtained was washed with methanol at 0°C, and then washed with water, and then the resulting mixture was dried to obtain a dibromo intermediate product ;
[0096] The electron-donating group-containing compound 4-diphenylaminostyrene (0.52nmol), the bis-bromo intermediate (0.52mmol), Pd(OAc) 2 (0.026mmol) and Ag 2 CO 3 (0.31 mmol) was placed in a 50 mL Schlenk test tube, added to 15 mL of toluene, and the reaction was st...
Embodiment 2
[0099] Prepare double bromine intermediate product according to the method of embodiment 1;
[0100] The electron-donating group-containing compound 4-methoxystyrene (1.04nmol), the bis-bromo intermediate (0.52mmol), Pd(OAc) 2 (0.052mmol) and Ag 2 CO 3 (0.62mmol) was placed in a 50mL Schlenk test tube, added to 15mL of toluene, stirred at 110°C for 18h, and the obtained product system was purified by silica gel column. The reagents used were dichloromethane and petroleum ether (volume ratio was 1:1), The cyanostyrene derivatives are obtained, and the structural formula is:
[0101] Record it as DOB.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com