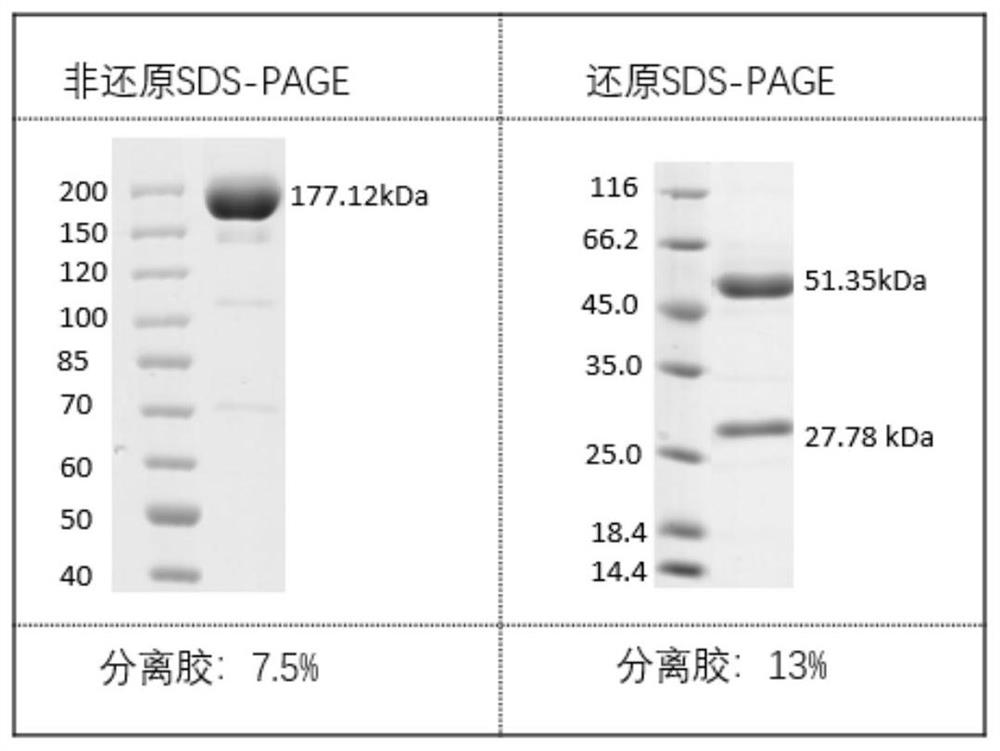
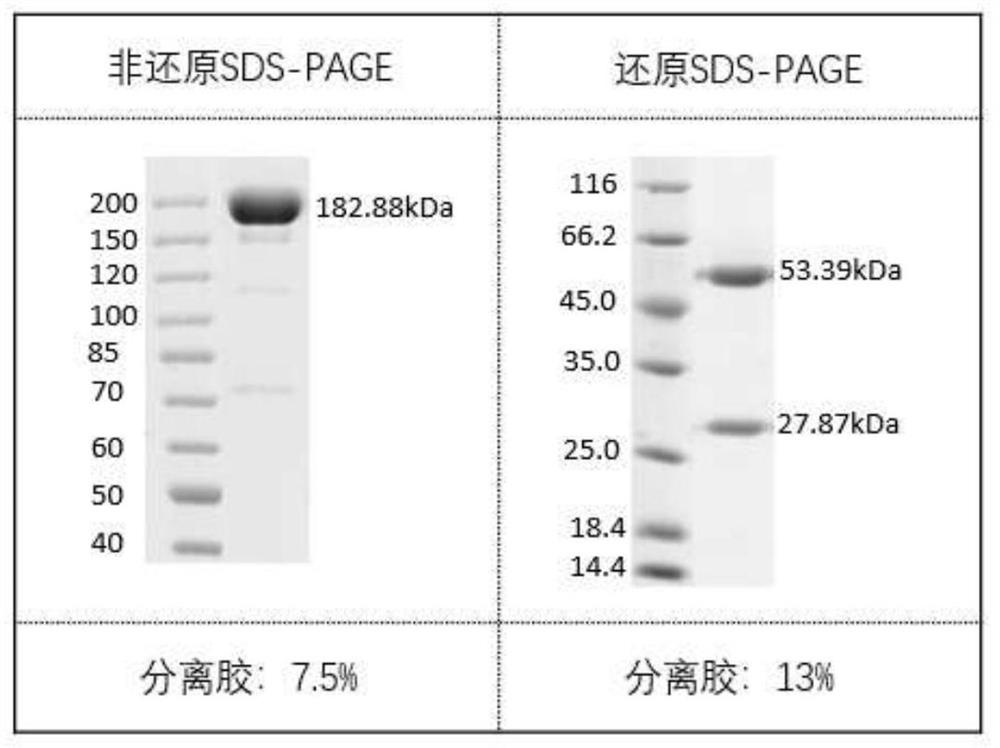
Antibodies against sars-cov-2 mutant strains and uses thereof
A sars-cov-2, antibody technology, applied in the direction of antibodies, applications, antiviral agents, etc., can solve the problems of vaccine effectiveness and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Example 1: Preparation of antibodies BD812 and BD836
[0153] 1.1 Acquisition of antibody sequences
[0154] 1) Isolation of B cells
[0155] Blood was collected from people infected with SARS-CoV-2 virus and recovered for more than half a year (provided by Beijing You'an Hospital), and peripheral blood mononuclear cells were extracted. Utilize STEMCELL EasySep TM The Human BCell Isolation Kit (STEMCELL, 17954) isolates B cells from peripheral blood mononuclear cells.
[0156] 2) Enrichment and sequencing of antigen-specific B cells
[0157] Combination of antigenic proteins (RBD, S1, S2) with streptavidin with DNA oligonucleotides and fluorescence: Combining RBD (Sino Biological 40592-V27H-B) with TotalSeq TM -C0951 PE Streptavidin (Biolegend 405261) was assembled into complexes according to the manufacturer's instructions, similarly S1 (Sino Biological 40591-V27H-B) and TotalSeq TM -C0952 PE Streptavidin (Biolegend 405263), S2 (SinoBiological 40590-V08B-B) TotalSeq...
Embodiment 2
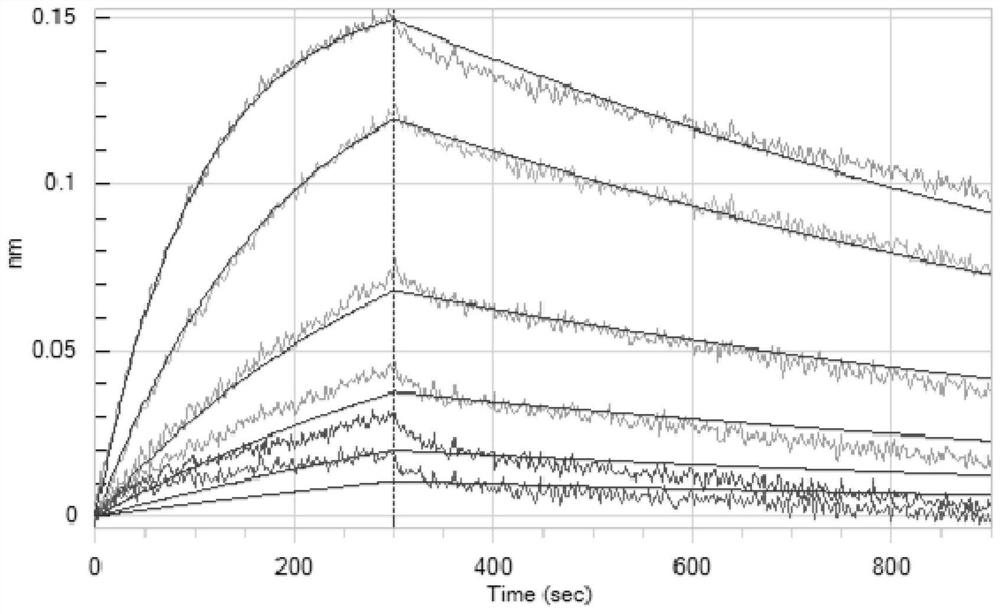
[0163] Example 2: Identification of Antigen Binding Ability of Antibodies BD812 and BD836
[0164] Recombinantly expressed RBD (Sinobiologicals, Cat: 40592-V08B, which contains the RBD fragment sequence YP_009724390.1 and His tag) was used as the coating antigen, and horseradish peroxidase (HRP)-labeled Goat Anti-Human IgG (HRP) was used as the coating antigen. +L) (Jackson, 109-036-088) was used as the secondary antibody to detect the specificity of the purified antigen by ELISA. Briefly, 96-well plates were coated with recombinantly expressed S protein RBD (the amino acid sequence of which is SEQ ID NO: 41), followed by blocking with blocking solution. Then, the monoclonal antibodies to be tested (irrelevant control antibodies, BD812, BD836) were added and incubated. After washing with ELISA wash solution, horseradish peroxidase-labeled Goat Anti-Human IgG (H+L) was added as secondary antibody (diluted at 1:5000), and the incubation was continued. The ELISA plate was washe...
Embodiment 3
[0170] Example 3: Assessment of the ability of antibodies BD812 and BD836 to neutralize pseudoviruses
[0171] The SARS-CoV-2 pseudovirus (mutant strain pseudovirus) used in this experiment was provided by the China National Institute for Food and Drug Control. The pseudovirus can simulate the cell infection characteristics similar to the true virus, and carry a fluorescein reporter gene for detection. The experimental steps can be referred to: Nie, J., Li, Q., Wu, J. et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat Protoc 15, 3699–3715 (2020). https: https: / / doi.org / 10.1038 / s41596-020-0394-5. Specific steps are as follows:
[0172] 1) The arrangement of samples in the 96-well plate is as follows: B2-G2 are cell control wells (CC), containing Huh-7 cells, without antibody samples and pseudoviruses; B3-G3 are virus control wells (VC), containing Huh-7 cells and pseudoviruses, without antibody samples; wells of B4-G11 are experi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com