Application of interleukin-37b in preparation of product for preventing and treating Kawasaki disease
A technology for interleukin and Kawasaki disease, applied in medical preparations containing active ingredients, cardiovascular system diseases, peptide/protein components, etc., to reduce endothelial cell apoptosis and inflammatory response, and alleviate coronary arteritis in Kawasaki disease Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 2ml blood samples were collected from each healthy control and acute KD patients, and then centrifuged to collect serum. The collected serum samples were routinely stored at -80°C within 4 hours after collection for later use. In addition, for all participants, written informed consent was given for their clinical information and blood samples for academic research. This study was commissioned by the Ethics Committee of Wenzhou Medical University and conducted in accordance with the Declaration of Helsinki.
[0069] The serum concentration of IL-37 was determined with the corresponding enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions. Human IL-37 ELISA kit was purchased from SolarBio (Beijing, China).
[0070] To preliminarily confirm that IL-37 may be involved in the pathological process of KD, we first detected the level of IL-37 in the serum of KD patients and compared it with healthy controls (HC). like figure 1 As show...
Embodiment 2
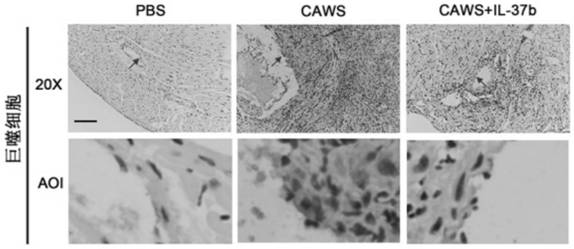
[0077] CAWS were prepared from Candida albicans NBRC1385. Briefly, Candida albicans was cultured in C-limiting medium at 27°C at 170 rpm for 2 days. Next, add an equal amount of ethanol and place in a refrigerator at 4 °C overnight. The precipitate was collected by centrifugation, dissolved in water, stirred for 2 h and then centrifuged again. Finally, the soluble fraction was obtained, and after adding an equal amount of ethanol, it was placed in a refrigerator at 4° C. overnight, and then centrifuged again. The resulting precipitate was collected with acetone, and after air drying, the resulting CAWS were dissolved in PBS buffer and autoclaved before use.
[0078] The KD animal model was 3-4 weeks old male C57BL / 6 mice, which were purchased from Wenzhou Medical University, certificate number: SCXK[ZJ]2005-0019. Guidelines for the Care and Use of Animals. The breeding temperature is 23±2°C, and the humidity is 50±5%. The mice were randomly divided into 3 groups: PBS grou...
Embodiment 3
[0086] According to the method of Example 1, KD patient serum (KD) and healthy control serum (HC) were obtained.
[0087] Human umbilical vein endothelial cells (HUVECs) and human monocytic leukemia cell line (THP1) were used as research objects. HUVECs and THP1 were identified by short tandem repeat (STR) markers by Gene Detection Biotech (Suzhou, China) and KeyCen Biotechnology (Nanjing, China) and were free of mycoplasma contamination. HUVEC and THP1 were routinely cultured according to previous reports. If necessary, human umbilical vein endothelial cells were pretreated with IL-37b protein for 1 h, and then co-cultured with THP1 cells for 24 h. All cultures were grown in healthy control serum or KD patient serum. Serum was diluted in DMEM medium.
[0088] To study the protective effect of IL-37b, we added different concentrations of IL-37b (0 ng / ml, 10 ng / ml and 100 ng / ml) to human umbilical vein endothelial cells and co-cultured them with KD serum-treated THP1 cells. U...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com