HUMANIZED ANTI-SIRPalpha ANTIBODIES
A humanized and antibody technology, applied in the direction of antibodies, antibody medical components, anti-tumor drugs, etc., can solve the problem of not promoting the anti-tumor activity of immune effector cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
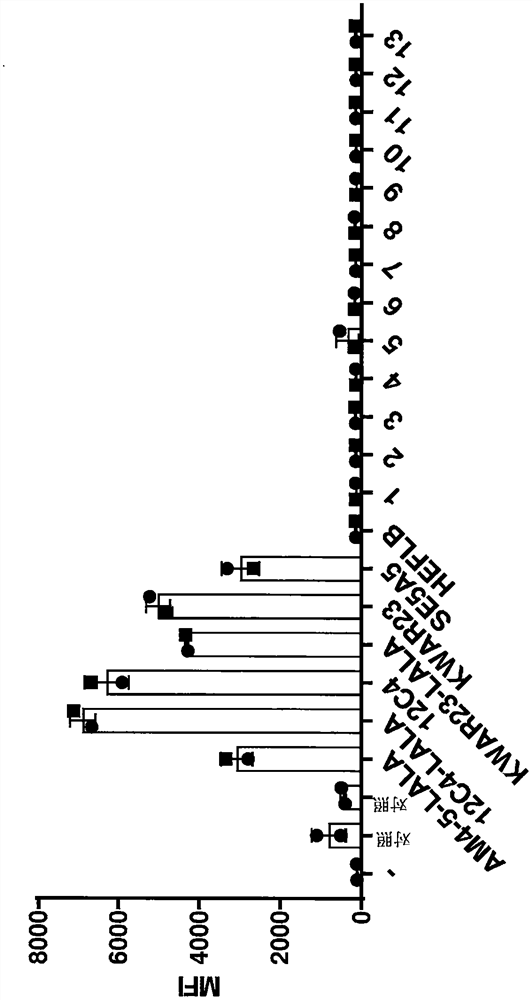
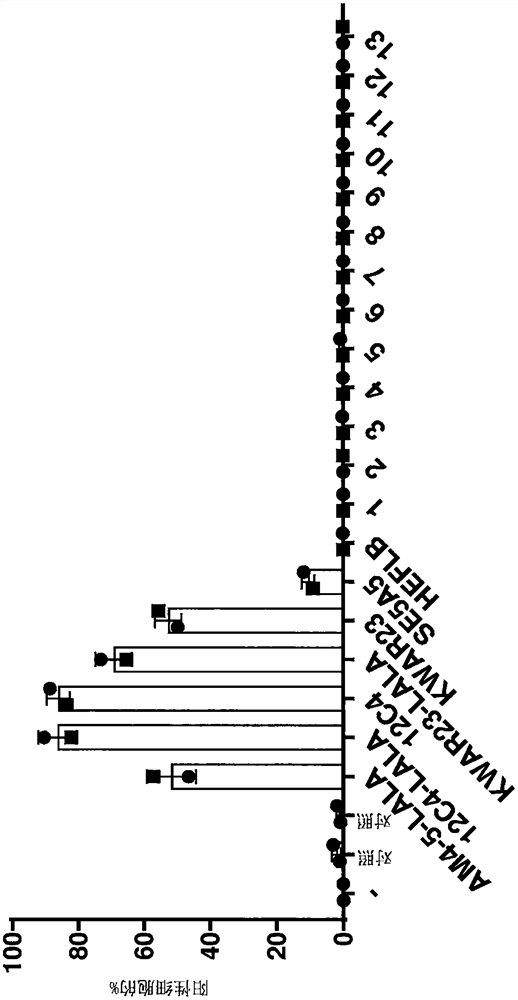
Image
Examples
Embodiment
[0169] 1. Transient Expression of Antibodies
[0170] a) Preparation of cDNA constructs and expression vectors
[0171] The heavy chain variable region (HCVR) amino acid sequences of the antibodies were each linked at the N-terminus to a leader sequence (SEQ ID NO: 28 for antibodies 1 to 13) and at the C-terminus to a human IgG according to SEQ ID NO: 25 1 HC LALA constant domain linkage (computer simulation). The HCVR amino acid sequences of antibodies 12C4, 12C4-LALA, 29AM4-5-LALA or KWAR23-LALA were each linked at the N-terminus to the HAVT20 leader sequence (SEQ ID NO:27) and at the C-terminus to the HAVT20 leader sequence (SEQ ID NO:25) human IgG 1 HC LALA or wild type human IgG 1 The constant domains of HC (SEQ ID NO: 24) are linked. KWAR23 has the standard adalimumab heavy chain constant domain but lacks the LALA mutation. The HEFLB heavy chain was IgG4 and was used as disclosed in SEQ ID NO: 42 of WO 2017 / 178653. The resulting amino acid sequence was back-trans...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com