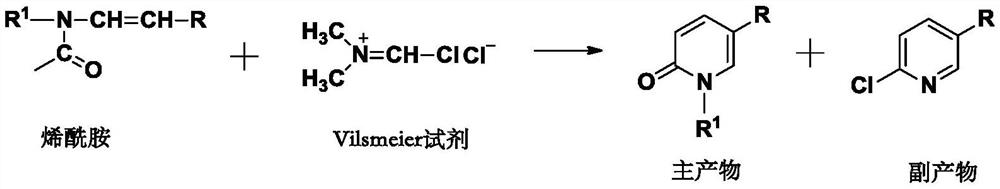
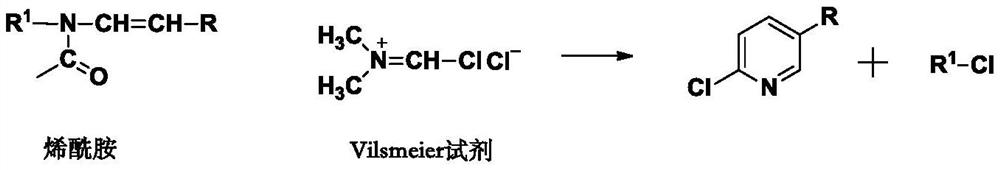
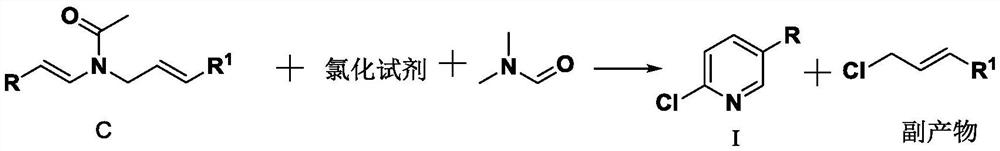
A kind of method for preparing 2-chloro-5-substituted pyridine
A technology of chloropyridine and chlorinating agents, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of large amount of pollutants, complicated operation, and complicated separation, so as to save equipment investment and simplify The effect of short operating procedures and synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Synthesis of N-propylene allylamine (R=methyl, R 1 =H)
[0045]
[0046] In a 500mL three-necked flask, add 57.7g (99.0%, 1.0mol, 1.0eq) of allylamine, cool the low temperature reaction bath to about 0°C, and slowly add 61.9g (98.5%, 1.05mol, 1.05eq) of propionaldehyde dropwise . After the dropwise addition was completed, 15 g of potassium hydroxide (85%, 0.23 mol, 0.23 eq) was added in batches after stirring at a temperature for 30 min, continued stirring for 2 h, and then allowed to stand for stratification. The water layer was separated to obtain 97.2 g of an organic layer (content 97%, yield 97%, 0.97 mol), which was a crude condensate product, pale yellow transparent liquid, which was directly used in the next reaction.
[0047] NMR data:
[0048] 1 H NMR (CDCl3) δ 1.03 (3H), 2.24 (2H), 3.90 (2H), 4.81-5.23 (2H), 5.58-6.26 (1H), 7.60 (1H).
[0049] With reference to the preparation method in Example 1, the following structural imines are prepared...
Embodiment 9
[0054] Example 9, Synthesis of N-allyl-N-propenylacetamide (R=methyl, R 1 =H, acetic anhydride)
[0055]
[0056] Add 70g (99.0%, 0.69mol, 0.7eq) of triethylamine and 121.2g (98.0%, 1.16mol, 1.2eq) of acetic anhydride to a 1000mL four-necked flask with a constant pressure dropping funnel, and cool the reaction bath at low temperature to At about 0°C, under stirring conditions, 97.0g (content 97%, 0.97mol, 1.0eq) of the crude condensate obtained in step 1 was slowly added dropwise to the reaction system, and the reaction was performed at about 25°C for 3h after the dropwise addition. After the reaction was completed, the low boiling point (the mixture of triethylamine and acetic acid) was removed by distillation under reduced pressure with a water pump, and the mixture was steamed until the internal temperature did not exceed 120°C. The distillation residue is the crude amide product, 135 g (content 90%, 0.87 mol, yield 87.4%), brown-red oil.
[0057] Referring to the meth...
Embodiment 16
[0061] Example 16, Synthesis of 2-chloro-5-methylpyridine (R=methyl, R 1 =H, the chlorinating agent is bis(trichloromethyl carbonate)
[0062]
[0063] 135g of crude amide product (content 90%, 0.87mol), 100mL of 1,2-dichloroethane, and 74.1g of DMF (99.0%, 1.0mol, 1.15eq) were successively added to a 1L reaction flask, stirred evenly, and a low temperature reaction bath was used. The temperature was lowered to about 0 °C, and then a solution of 191.2 g (99.0%, 0.64 mol, 0.73 eq) of bis(trichloromethyl carbonate) and 400 mL of 1,2-dichloroethane was slowly added dropwise with a constant pressure dropping funnel. In the process of adding, the internal temperature should not exceed 20 °C. After dripping, slowly heat up to about 40 °C for 2 hours, then remove most of the solvent by vacuum distillation, when the temperature in the kettle reaches about 100 °C (the solvent 1,2-dichloroethane has been distilled off at this time), Switch the receiving flask, collect 2-chloro-5-me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com