A kind of chiral synthesis method of dorzolamide hydrochloride
A technology for chiral synthesis of dorzolamide hydrochloride, which is applied in the field of chiral synthesis of dorzolamide hydrochloride, can solve problems such as the lack of chiral selectivity of reaction products, and achieve simple synthesis methods, strong chiral selectivity, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The chiral synthesis method of dorzolamide hydrochloride in this embodiment comprises the following steps:
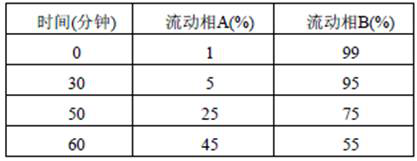
[0038] (1) At 0°C, add 12L of ethanol to a 20L reaction kettle, then add 2.95 kg (10.0 mol) of compound I and 495 g (11 mol) of ethylamine, then raise the temperature to 20°C, imidize for 10 hours, TLC Monitor the reaction, cool to room temperature, concentrate the organic phase under reduced pressure (0.2 mmHg), and recrystallize with toluene to obtain 2.83 kg of compound II (8.8 mol, molar yield: 88%), with a purity of 97.4% by HPLC;
[0039] (2) Add 12L of ethanol to the 20L autoclave, then add 2.83kg (8.8mol) of compound II and 100g of water obtained in step (1), and then add 45g (0.09mol) of catalyst bis(1,5-cyclo Octadiene) iridium(I) tetrafluoroborate and 90 g (0.18 mol) of the chiral ligand (R)-(+)-1,1'-binaphthyl-2'-isopropoxy-2-diphenylphosphine , heated to 40°C, carried out asymmetric hydrogenation reaction under 1Mpa hydrogen pressure for 4 h, monito...
Embodiment 2
[0046] The chiral synthesis method of dorzolamide hydrochloride in this embodiment comprises the following steps:
[0047] (1) At 0°C, add 12L of isopropanol to a 20L reaction kettle, then add 2.95 kg (10.0 mol) of compound I and 495 g (11 mol) of ethylamine, and imidize for 14 hours, monitor the reaction by TLC, and cool After reaching room temperature, the organic phase was concentrated under reduced pressure (0.2 mmHg), and then recrystallized with toluene to obtain 2.86 kg of compound II (8.90 mol, molar yield of 89.0%), with a purity of 98.2% by HPLC;
[0048](2) Add 12L of isopropanol to a 20L autoclave, then add 2.86kg (8.9mol) of compound II and 100g of water obtained in step (1) in sequence, and then add 19.9g (0.05mol) of catalyst 1,5-cyclooctyl Diene (acetylacetonate) iridium(I) and 49.6 g (0.1 mol) of the chiral ligand (R)-(+)-1,1'-binaphthyl-2'-isopropoxy-2-diphenylphosphine , heated up to 50°C, carried out asymmetric hydrogenation reaction under 1Mpa hydrogen pr...
Embodiment 3
[0051] The chiral synthesis method of dorzolamide hydrochloride in this embodiment comprises the following steps:
[0052] (1) At 0°C, add 12L tetrahydrofuran to a 20L reaction kettle, then add 2.95 kg (10.0 mol) of compound I and 540 g (12 mol) of ethylamine, then control the temperature to -10°C, and imidize for 14 hours , the reaction was monitored by TLC, cooled to room temperature, the organic phase was concentrated under reduced pressure (0.2 mmHg), and then recrystallized with toluene to obtain 2.93 kg of compound II (9.1 mol, molar yield 91%), with a purity of 97.8% by HPLC ;
[0053] (2) Add 12L of sec-butanol to a 20L autoclave, then add 2.93kg (9.1mol) of compound II and 100g of water obtained in step (1) in sequence, and then add 45.7g (0.09mol) of catalyst 1,5-cyclooct Diene (hexafluoroacetylacetonate) iridium (I) and 89.3 g (0.18 mol) of the chiral ligand (R)-(+)-1,1'-binaphthyl-2'-isopropoxy-2-di For phenylphosphine, heat up to 60°C, carry out asymmetric hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com