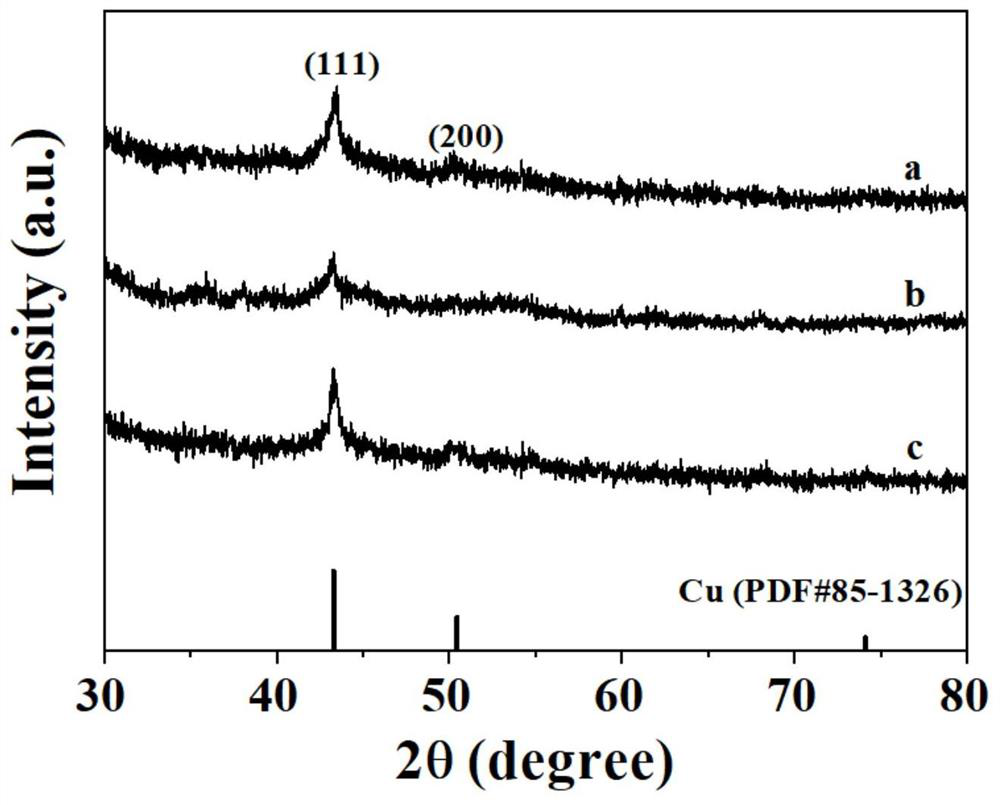
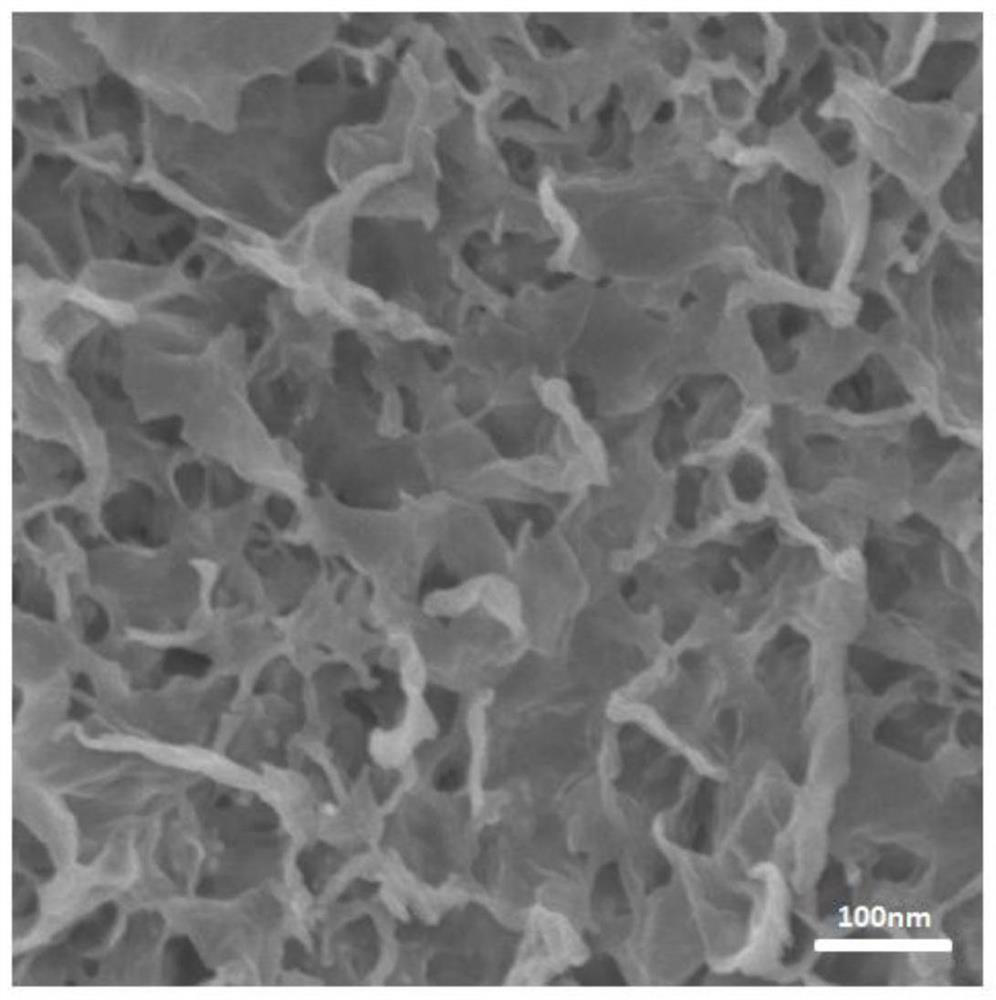
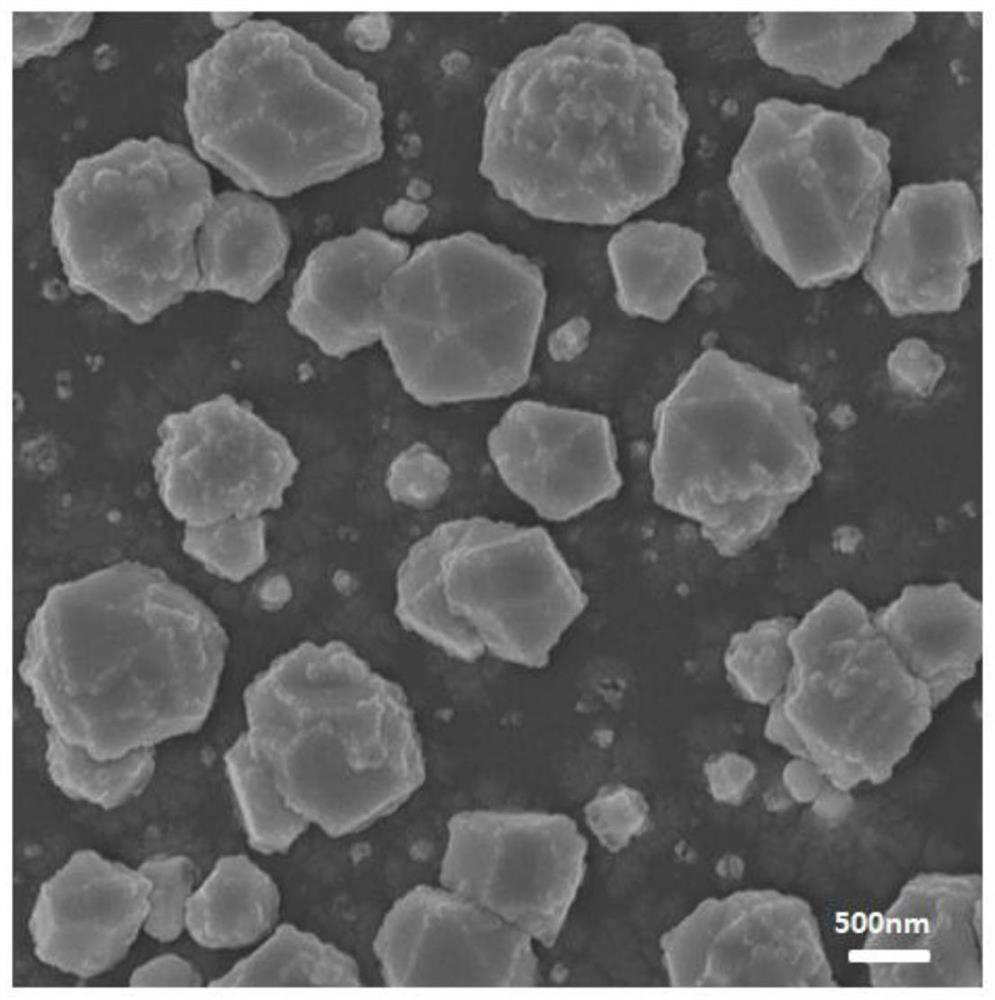
Preparation method and application of silver-doped copper nanosheet catalyst
A catalyst and copper nanotechnology, which is applied in the field of preparation of silver-doped copper nanosheet catalysts, can solve the problems of low catalyst selectivity, low catalytic yield, and low Faradaic efficiency, and achieve clean and pollution-free products and improved selectivity , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 0.8~1.6g of CuSO 4 ·5H 2 O and 20-50 mg of 3,5-diamino-1,2,4-triazole (DAT), which were dissolved in 15-20 ml deionized water respectively; then the two reactant solutions were mixed and stirred evenly, and Add 0~0.5mM AgNO to the mixed solution 3 solution, after stirring well, use 1MH 2 SO 4 Adjust the pH of the solution to 2-3; then place the mixed solution in a three-electrode system, use carbon paper as the working electrode, Ag / AgCl electrode as the reference electrode, and graphite electrode as the counter electrode, and use the chronoamperometry for electrodeposition of the catalyst , set the deposition voltage to -2~-4mA, and the deposition time to 0.5~2min to obtain Ag-Cu / CP DAT composite material.
Embodiment 2
[0044] Adopt the Ag-Cu / CP that embodiment 1 prepares respectively DAT Ag-Cu / CP prepared by composite material, comparative example 1 DAT The Ag-Cu / CP composite material prepared in Comparative Example 2 was used as a catalyst for electrochemical synthesis of ammonia.
[0045] The electrochemical ammonia synthesis reaction is carried out in an H-type electrolytic cell. The working electrode is carbon paper loaded with catalyst and the Ag / AgCl electrode as the reference electrode is in the same room, and the platinum sheet electrode is in the other room as the counter electrode. The protons are separated by a proton exchange membrane. Add an equal amount of 0.1M Na to the two chambers 2 SO 4 As the electrolyte, high-purity argon and high-purity nitrogen are passed into the electrolyte for 30 minutes before the electrochemical nitrogen reduction to synthesize ammonia to remove impurity gases in the electrolyte. Chronovoltage method was used to carry out nitrogen reduction syn...
Embodiment 3
[0047] Weigh 0.45-0.9g of NaOH, dissolve it in 5-10mL of deionized water, add 10-20mL of sodium hypochlorite to the solution after fully dissolving, and stir well to obtain color solution a; weigh 1-2g of salicylic acid and Dissolve 0.8-1.6g of NaOH in 20-40mL of deionized water, and obtain color developing solution b after fully dissolving; weigh 0.1-0.2g of nitroprusside and dissolve in 10-20mL of deionized water, and obtain color developing solution after fully dissolving liquid c.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com