Human growth differentiation factor-15 magnetic particle chemiluminescence detection kit and application thereof
A technology for detection of growth differentiation factors and chemiluminescence, which is applied in the field of medicine, can solve the problems of ELISA detection method, such as long time-consuming process, inability to realize rapid and large-scale detection, and many manual operations, to achieve improved sensitivity, small magnetic particles, and improved sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
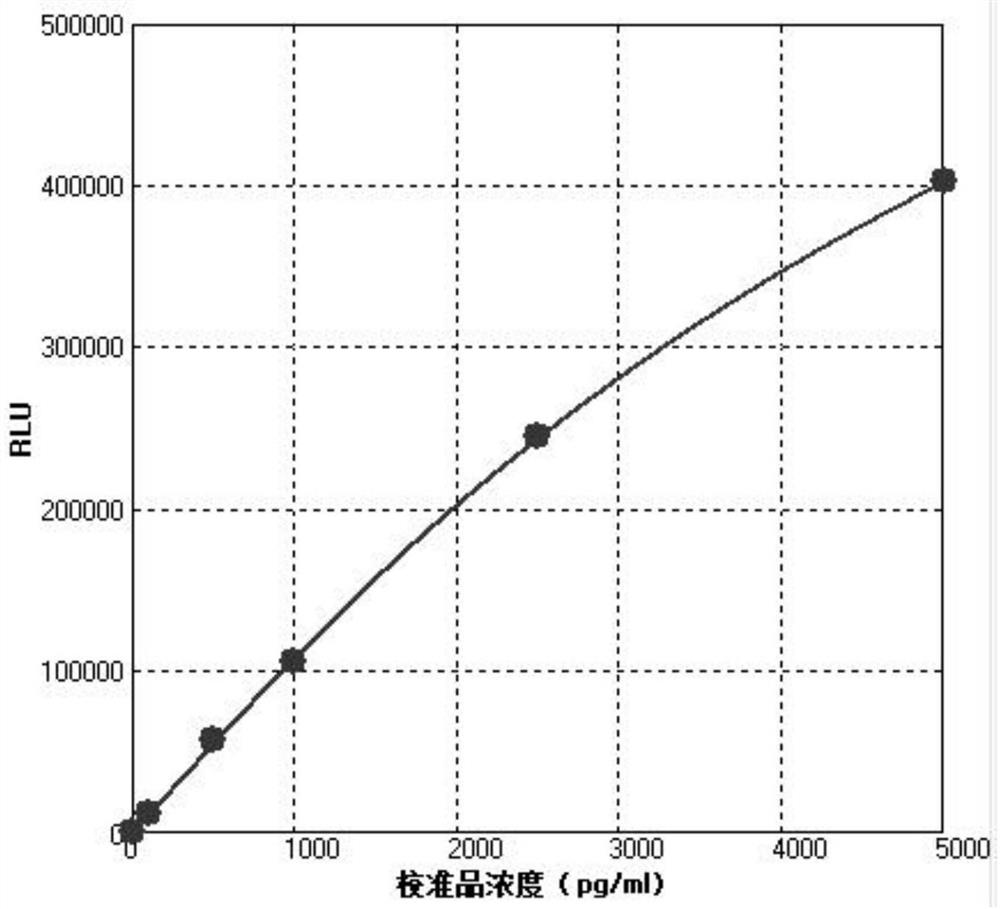
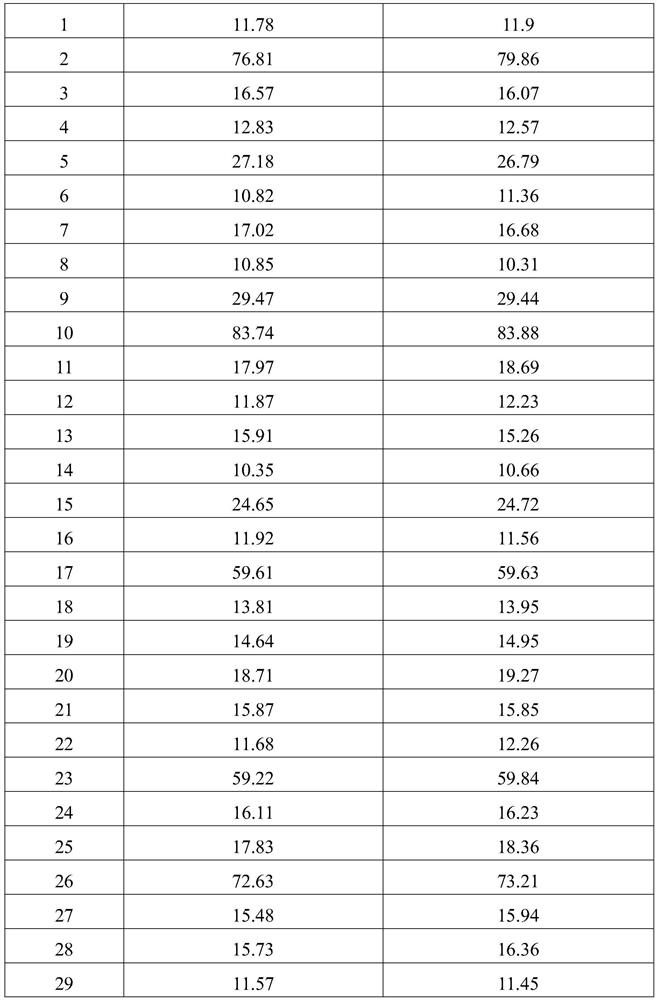
Image
Examples
Embodiment 1
[0073] Example 1. Preparation of magnetic particle chemiluminescence detection kit for detection of human growth differentiation factor-15 and establishment of detection system
[0074] 1. Experimental materials: mouse anti-human GDF-15 monoclonal antibody [23G10.F8], [7C12.B3.F2] (purchased from ABCAM Company, article number: ab105732, ab106112), recombinant human GDF-15 antigen (purchased from ABCAM company, catalog number: ab50077).
[0075] 2. Preparation of FITC-labeled GDF15 monoclonal antibody 1
[0076] Main materials: fluorescein 5(6)-isothiocyanate (FITC); GDF15 monoclonal antibody 1 [23G10.F8]; sodium bicarbonate.
[0077] Preparation Process:
[0078] (1) Elute through the PD-10 column, replace the buffer of GDF15 monoclonal antibody 1 with 200mmol / L sodium bicarbonate buffer at pH9.0, and dilute the antibody to 3mg / ml.
[0079] (2) Put the antibody into a dialysis bag, put it into a pH 9.0 sodium bicarbonate buffer solution containing 0.5 mg / mL FITC, and react ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com