Biphenyl compound and diterpenoid compound as well as preparation method and application thereof
A kind of technology of compound and diterpenoid, applied in the field of biphenyl compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation of embodiment 1 biphenyl compound and diterpenoid compound
[0056] 1. Preparation of strain fermentation broth: inoculate the strain into biomalt extract (biomalt) agar (agar) medium (3% biomalt, 2% agar), and ferment at 28° C. for 28 days.
[0057] 2. Preparation of total crude extract
[0058] 20L of the fermentation liquid of the strain was subjected to conventional ethyl acetate ultrasonic extraction, and the extract was concentrated under reduced pressure to obtain 13.6 g of the total crude extract.
[0059] 3. Separation and purification
[0060] The crude extract was subjected to normal-phase silica gel column chromatography (200-300 mesh), and gradient elution was carried out with dichloromethane / methanol system (v / v 100:0, 100:1, 80:1, 60:1, 40:1 , 30:1, 20:1, 10:1, 4:1), the fractions were collected and combined by TLC analysis to obtain 13 fractions (Fr.1~Fr.13).
[0061] Component Fr.2 (52.7mg) was subjected to Sephadex LH-20 gel column c...
Embodiment 2
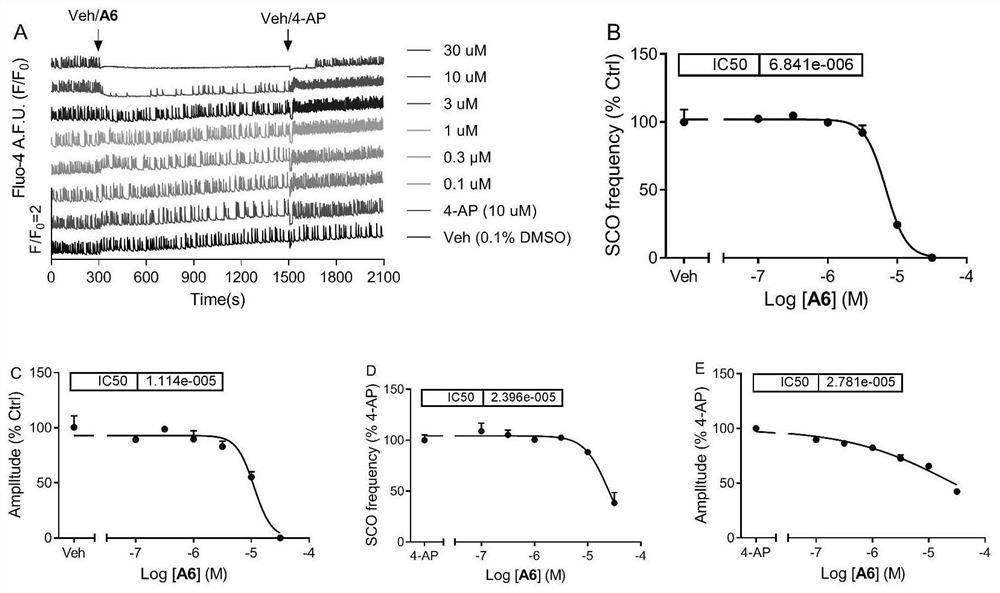
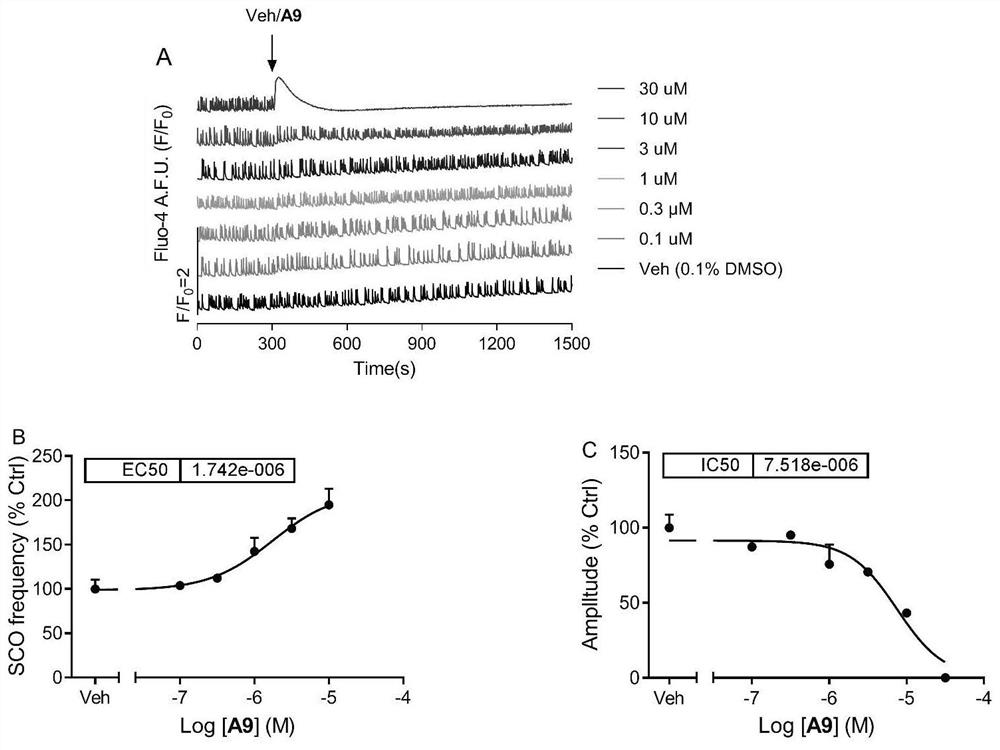
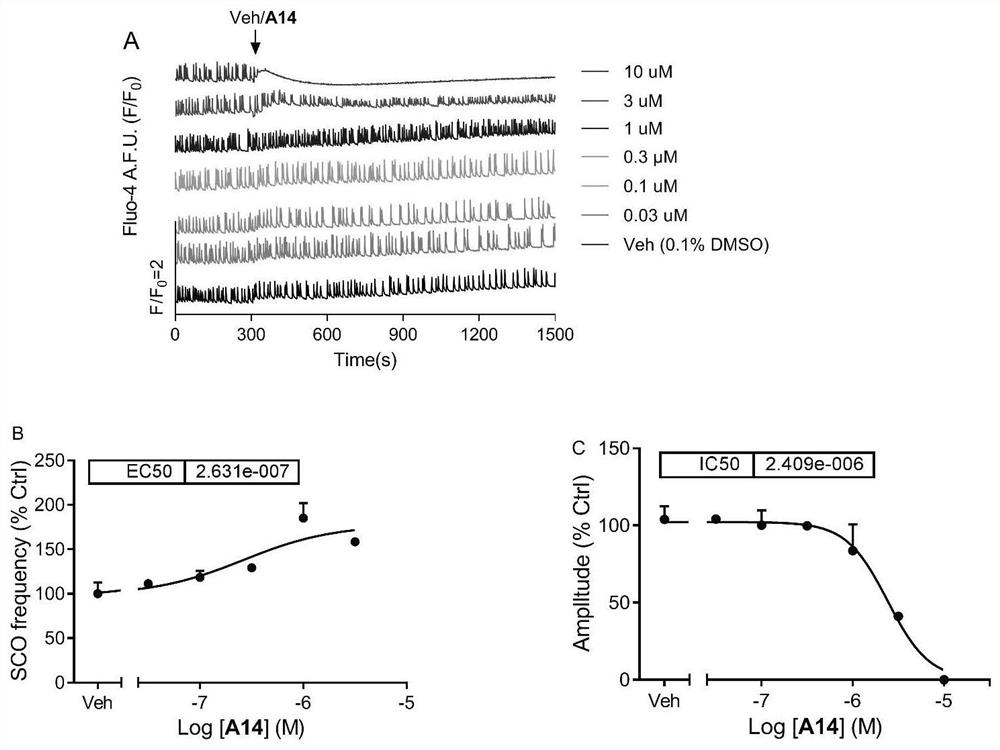
[0128] Example 2 Effects of Compounds of the Present Invention on the Spontaneous Synchronous Calcium Oscillation of Mouse Primary Cortical Neurons
[0129] 1. Isolation and culture of mouse cerebral cortex neurons
[0130] For the isolation and culture of C57Bl / 6J mouse cerebral cortex neurons, see references (Zheng, J.; Yu, Y.; Feng, W.; Li, J.; Liu, J.; Zhang, C.; Dong, Y. .; Pessah, IN.; Cao, Z. Environmental Health Perspectives 2019, 127, 67003). The 0-1 day old suckling mice were taken out, their heads were decapitated, and their brains were taken. The cerebral cortex was separated under a dissecting microscope, and the meninges were carefully peeled off. After being blown away with a Pasteur pipette, trypsin was digested at 37°C for 25 minutes. In the dissection buffer containing trypsin inhibitor (soybean) and deoxyribonuclease I, the cortex was blown into a single cell suspension, and the cell suspension was centrifuged (1000rmp / 5min). Resuspend with Neuron Plating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com