Quality control preparation method for cell staining
A quality control product and cell technology, applied in the field of quality control product preparation for cell staining, can solve the problem of low accuracy of circulating tumor cells in lung cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
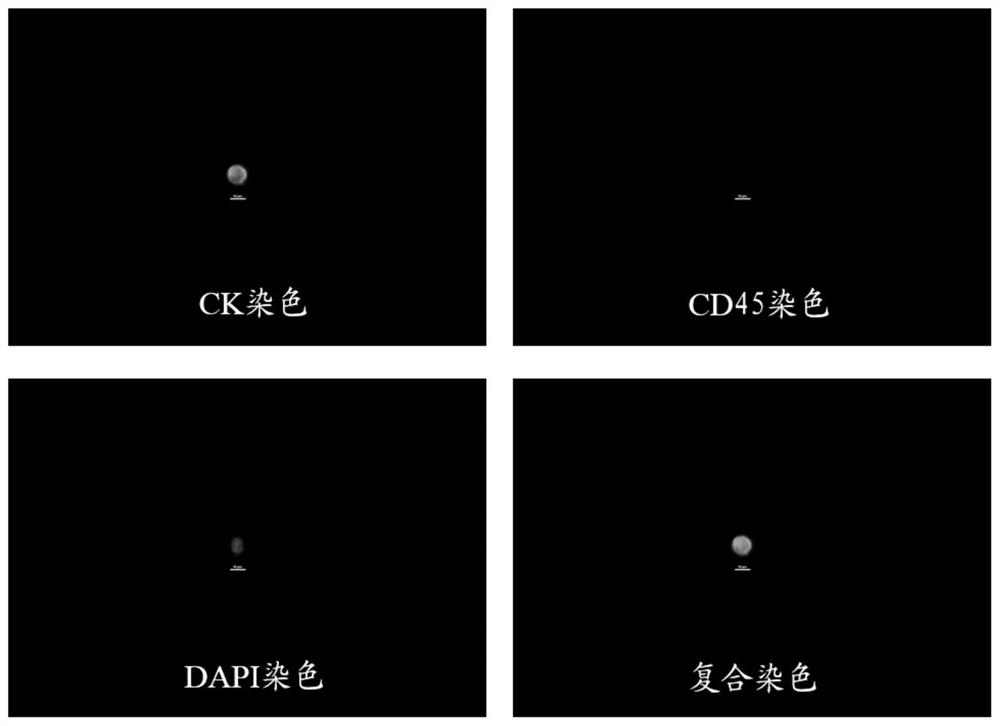
[0032] The present invention proposes a method for preparing a quality control product for cell staining, comprising the following steps: (1) E-Cadherin immunomagnetic beads are mixed with a binding buffer to obtain E-Cadherin immunomagnetic beads working solution, and CEA immunomagnetic beads are mixed with The binding buffer is mixed to obtain the CEA immunomagnetic beads working solution, and the CD45 immunomagnetic beads are mixed with the binding buffer to obtain the CD45 immunomagnetic beads working solution; (2) the E-Cadherin immunomagnetic beads working solution, the CEA Mixing the immunomagnetic bead working solution, the CD45 immunomagnetic bead working solution, leukocytes and lung cancer circulating tumor cells to obtain the first mixed solution; (3) removing the liquid in the first mixed solution, adding a fixative to fix it, and obtaining Quality control of circulating tumor cells in lung cancer.
[0033] In the preparation method of the present invention, E-cad...
Embodiment 1
[0043] 1. Mix white blood cells with NCI-H1993 cells (number ratio: 1:10), the total volume is 700ul;
[0044] 2. Take 30ul of E-Cadherin immunomagnetic beads, add 1ml of binding buffer, discard the supernatant, and resuspend the magnetic beads with 1ml of binding buffer to make E-Cadherin immunomagnetic beads working solution;
[0045] 3. Treat CEA immunomagnetic beads and CD45 immunomagnetic beads with the same method as step 2 to make CEA immunomagnetic beads working solution and CD45 immunomagnetic beads working solution;
[0046] 4. Add 30ul of the E-Cadherin immunomagnetic bead working solution, 40ul of the CEA immunomagnetic bead working solution, and 50ul of the CD45 immunomagnetic bead working solution into the cell mixture in step 1, and mix evenly by inversion. Discard the supernatant and repeat the operation 3 times;
[0047] 5. Rotate and mix the centrifuge tube in a refrigerator at 4°C for 2 hours at a rotation speed of 15 rpm;
[0048] 6. After rotating and mi...
Embodiment 2
[0056] 1. Mix white blood cells with NCI-H1993 cells (the ratio of cell number is 10:1), and the total volume is 700ul;
[0057] 2. Take 30ul of E-Cadherin immunomagnetic beads, add 1ml of binding buffer, discard the supernatant, and resuspend the magnetic beads with 1ml of binding buffer to make E-Cadherin immunomagnetic beads working solution;
[0058] 3. Treat CEA immunomagnetic beads and CD45 immunomagnetic beads with the same method as step 2 to make CEA immunomagnetic beads working solution and CD45 immunomagnetic beads working solution;
[0059] 4. Add 40ul of the E-Cadherin immunomagnetic bead working solution, 30ul of the CEA immunomagnetic bead working solution, and 28ul of the CD45 immunomagnetic bead working solution into the cell mixture in step 1, and mix evenly by inversion. Discard the supernatant and repeat the operation 3 times;
[0060] 5. Rotate and mix the centrifuge tube in a refrigerator at 4°C for 2 hours at a rotation speed of 15 rpm;
[0061] 6. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com