Method for culturing pluripotent stem cells
A technology of pluripotent stem cells and culture methods, applied in the field of pluripotent stem cell culture, can solve the problems of inability to accurately evaluate cell growth and differentiation, inability to carry out large-scale drug screening, and high cost of suspension culture systems, so as to reduce the differences between experiments , saving cost, and stabilizing multiplication effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] In this example, the dynamic suspension culture of human embryonic stem cells H9 was carried out, and relevant detection was carried out on the cultured cells.
[0071] Dynamic suspension culture of pluripotent stem cells:
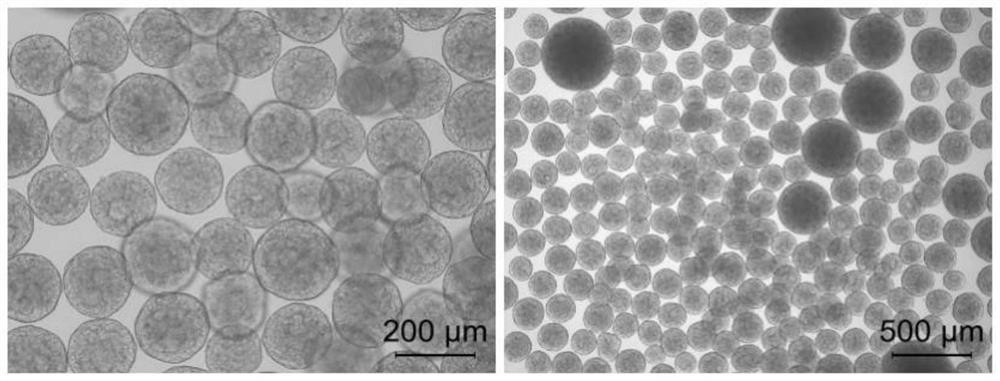
[0072] (1) with 5.5×10 5 Inoculation density of cells / mL Human embryonic stem cells H9 were inoculated in a 48-well plate, and dynamic suspension culture was carried out on an orbital shaker. ±5%, 5% CO 2 (v / v); fresh medium was replaced every 24 hours of culture, co-cultured for 4 days, the particle size of cell clusters was 300-400 μm, and the cell aggregation morphology was shown in figure 1 shown.
[0073] (2) Take out the cell suspension, pass through a 37 μm reversible filter to enrich the cell cluster (remove single cells), use 16 mL of Accutase (StemCell) to backwash the cell cluster into a centrifuge tube and place it in a 37°C water bath for 15 min. Pipet once every 5 minutes until cell flocculation appears, add 2 times the volume of m...
Embodiment 2
[0082] In this example, induced pluripotent stem cells (iPS) are dynamically suspended and cultured, and relevant tests are performed on the cultured cells. Except for the step (1) in the suspension culture, other steps and detection methods are the same as in Example 1. Step (1) in the present embodiment suspension culture is:
[0083] Take 5.5×10 5 Inoculation density of cells / mL Inoculated induced pluripotent stem cells (iPS) in 48-well plates, and carried out dynamic suspension culture on an orbital shaker, the medium was mTeSR1 complete medium, the culture system was 600 μL, the rotation speed was 180 rpm, and the temperature was 37°C , relative humidity 90±5%, 5% CO 2 (v / v); fresh medium was replaced every 24 hours of culture, and co-cultivated for 4 days.
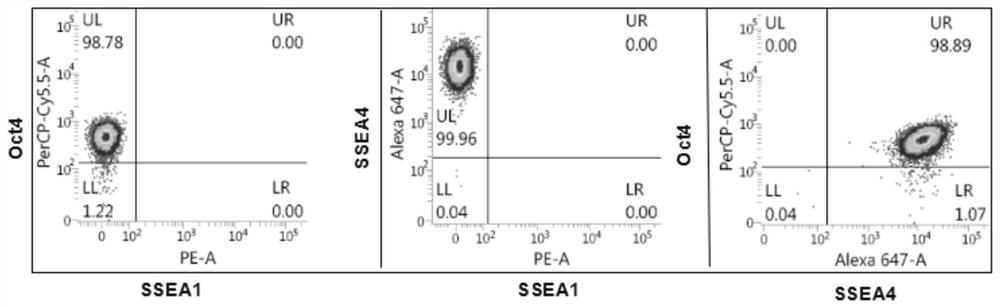
[0084] The aggregation morphology of the cells obtained through step (1) culture Figure 5 Shown; flow detection results see Image 6 shown, according to Image 6 , after being cultured by the method of the pre...
Embodiment 3
[0086] In this example, the dynamic suspension culture of human embryonic stem cells H9 was carried out, and relevant tests were carried out on the cultured cells:
[0087] Take 4.5×10 5 Inoculation density of cells / mL Human embryonic stem cells H9 were inoculated in a 48-well plate, and dynamic suspension culture was carried out on an orbital shaker. ±5%, 5% CO 2 (v / v); fresh medium was replaced every 24 hours of culture, and co-cultivated for 4 days.
[0088] The aggregation morphology of cultured cells is shown in Figure 8 As shown, it can be seen that the cell aggregates are uniform in size and round in shape.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com