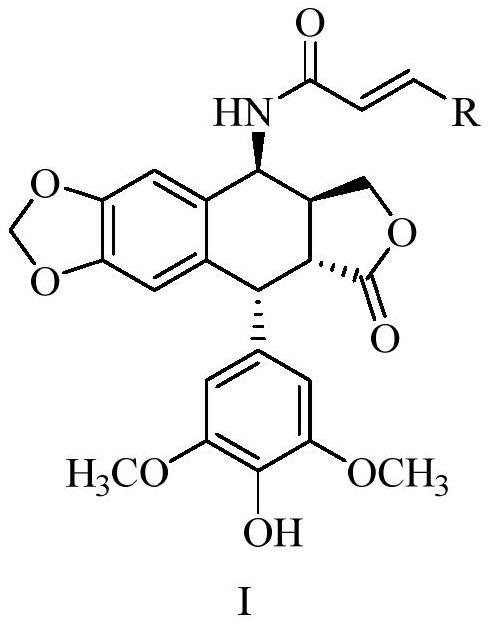
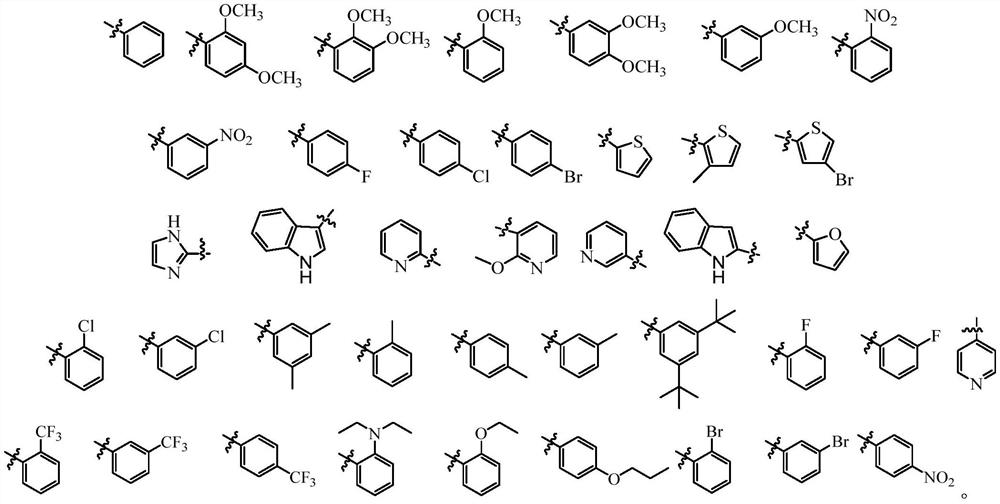
Epipodophyllotoxin derivatives, preparation method and application in the preparation of antitumor drugs
A technology for epipodophyllotoxin and anti-tumor drug, applied in the field of medicine, can solve the problems of toxic and side reactions, narrow anti-tumor spectrum, etc., and achieves simple and controllable preparation method and post-processing, good tumor inhibitory activity, and good market. The effect of developing prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
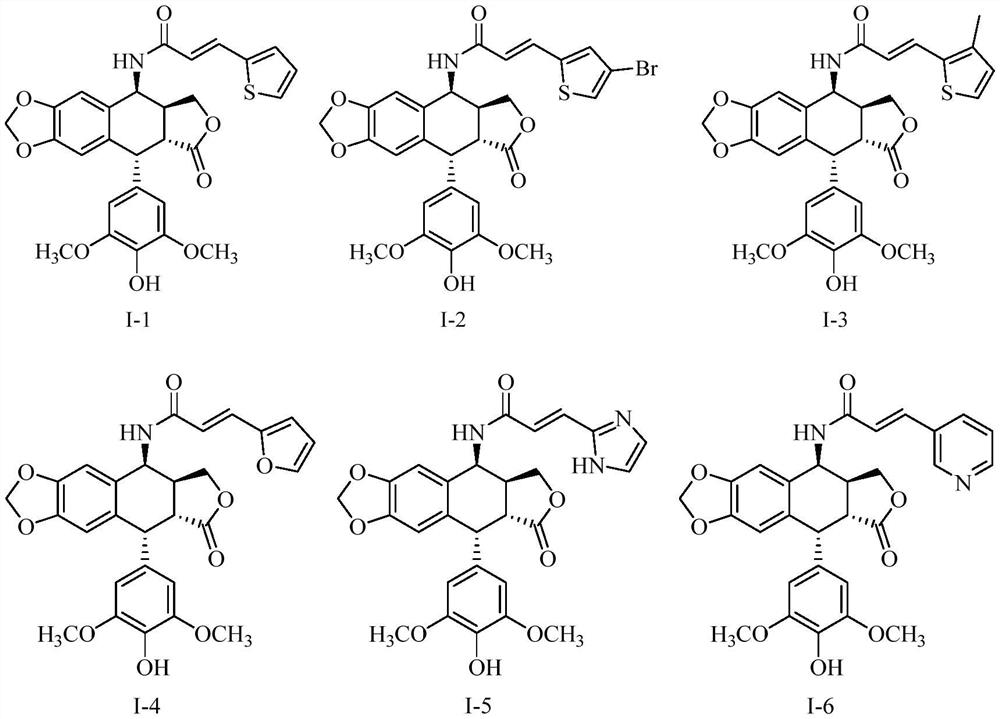
[0069] Preparation of 4β-4-(2"-thiopheneacrylamide)-4-deoxy-4'-desmethyl epipodophyllotoxin (I-1)
[0070]
[0071] 3-(2-thienyl)acrylic acid (1.15 mmol) was fed and post-treated according to the proportion of method 2 to prepare compound I-1, 181 mg of white solid, with a yield of 29.42%.
[0072] ESI-MS m / z:536,[M+H] + ;558,[M+Na] +
[0073] 1 H-NMR (400MHz, CDCl 3 )δppm: 7.66-7.62 (m, Ar-H, 2H), 7.56 (d, J = 15.6Hz, Hb, 1H), 7.15-7.13 (m, Ar-H, 1H), 6.84 (d, J = 15.6 Hz,1H),6.80(s,Ar-H,DMEP-H,1H),6.55(s,Ar-H,DMEP-H,1H),6.03-6.05(s,H-2',6',2H ),5.98((d,J=6.0Hz,OCH 2 O, 2H), 5.72(d, J=7.6Hz, NH, heavy water not exchanged, 1H), 5.41-5.44(s, m, H4+OH, OH heavy water exchanged, 2H), 4.62(d, J=4.8Hz ,1H),4.45-4.49(m,DMEP-H,H11a,1H),3.85-3.90(m,DMEP-H,H11b,1H),3.80(s,OCH 3 ,6H),3.40-3.44(m,DMEP-H,H3,1H),2.88-2.93(m,DMEP-H,H2,1H).
Embodiment 2
[0075] Preparation of 4β-4-((4”-bromo)-2”-thiopheneacrylamide)-4-deoxy-4′-desmethyl epipodophyllotoxin (I-2)
[0076]
[0077] 3-(4-bromo-2-thienyl)acrylic acid (1.15mmol) and 4β-amino-4'-desmethyl epipodophyllotoxin II (1.15mmol) were fed and post-treated according to method 2 to prepare compound I -2, white solid 275 mg, yield 39.01%.
[0078] ESI-MS m / z:614,[M+H] + ;636,[M+Na] +
[0079] 1 H-NMR (400MHz, CDCl 3 )δppm: 7.50-7.58 (m, Ar-H, 2H), 7.55 (d, J = 15.6Hz, Hb, 1H), 6.84 (d, J = 15.6Hz, 1H), 6.81 (s, Ar-H, DMEP-H,1H),6.54(s,Ar-H,DMEP-H,1H),6.02-6.04(s,H-2',6',2H),5.97((d,J=6.0Hz,OCH 2 O, 2H), 5.74(d, J=7.6Hz, NH, heavy water not exchanged, 1H), 5.41-5.44(s, m, H4+OH, OH heavy water exchanged, 2H), 4.62(d, J=4.8Hz ,1H),4.34-4.39(m,DMEP-H,H11a,1H),3.75-3.80(m,DMEP-H,H11b,1H),3.73(s,OCH 3 ,6H),3.40-3.45(m,DMEP-H,H3,1H),2.93-2.98(m,DMEP-H,H2,1H).
Embodiment 3
[0081] Preparation of 4β-4-((3”-methyl)-2”-thiopheneacrylamide)-4-deoxy-4′-desmethyl epipodophyllotoxin (I-3)
[0082]
[0083] 3-(3-methyl-2-thienyl)acrylic acid (1.15mmol) and 4β-amino-4'-desmethyl epipodophyllotoxin II (1.15mmol) were fed and post-treated according to method 2 to prepare the compound I-3, white solid 255 mg, yield 40.39%.
[0084] ESI-MS m / z:550,[M+H] + ;574,[M+Na] +
[0085] 1 H-NMR (400MHz, CDCl 3 )δppm: 7.66 (d, Ar-H, 1H), 7.55 (d, J = 15.6Hz, Hb, 1H), 7.11 (m, Ar-H, 1H), 6.84 (d, J = 15.6Hz, 1H) ,6.81(s,Ar-H,DMEP-H,1H),6.57(s,Ar-H,DMEP-H,1H),6.28(s,H-2',6',2H),5.99((d ,J=6.0Hz,OCH 2 O, 2H), 5.72(d, J=7.6Hz, NH, heavy water not exchanged, 1H), 5.38-5.42(s, m, H4+OH, OH heavy water exchanged, 2H), 4.57(d, J=5.2Hz ,1H),4.35-4.39(m,DMEP-H,H11a,1H),3.75-3.80(m,DMEP-H,H11b,1H),3.65(s,OCH 3 ,6H),3.38-3.44(m,DMEP-H,H3,1H),2.96-3.05(m,DMEP-H,H2,1H),2.26(s,CH3,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com