Halogenated nitrogen-containing heterocyclic matrine derivative as well as preparation method and application thereof
A nitrogen heterocycle and matrine technology is applied in the field of halogenated nitrogen-containing heterocycle matrine derivatives and their preparation, which can solve the problems of slow drug effect, low activity, low bioavailability and the like, and achieve fast drug effect , High bioavailability, good poisoning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7
[0064] This example provides a series of halogenated chiocyclic alkali derivatives, structural formulas as follows:
[0065]
[0066] Among them, MAT is a bitter base, 3-Cl-Pyr-MAT is 3-chloropyrazole alcoholica, 3-Br-Pyr-Mat is 3-bromipatezole, 3-I-Pyr-Mat is 3-iodopyrazole, 4-F-IND-MAT is 4-fluoronone bitter, 5-Cl-Ind-MAT is 5-chloroanne bitter, 5-Br-Ind-Mat For 5-bromohae borne, 5-Br-IND-MAT is 5-bromoblane alcoholic.
[0067] The above derivatives were prepared by the following procedure: dissolved the raguase and the halogenic heterocyclic lysate in 4.0 ml of organic solvent (ethanol, methanol, dioxane, acetonitrile, petroleum ether, etc.), via 0.03 g of Catalyst (cesium fluoride, cesium carbonate, tripassate, tetramethoxysilane, etc.), 2-10 h at a certain temperature (60-140 ° C), then subtracted analysis, eluent is acetate Esters, ethanol, methanol, and acetonitrile or the like are eluted in a certain volume ratio. Finally, the split segment obtained was detected by TLC, ...
Embodiment 1
[0069] Example 1, 3-Cl-Pyr-Mat spectrum data:
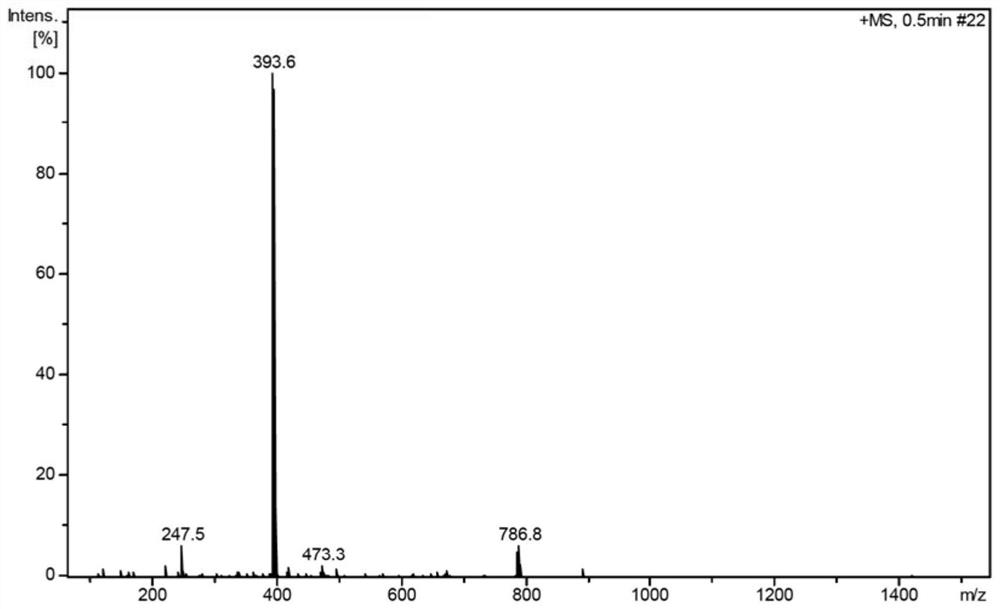
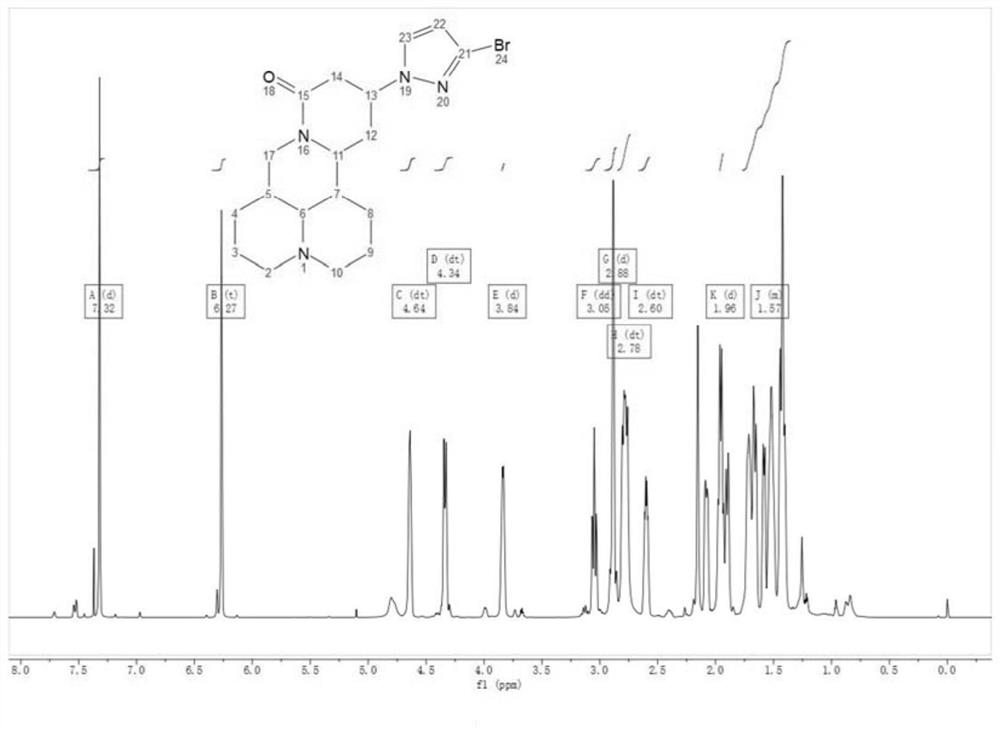
[0070] SELECTED IR DATA (KBR Disk, CM -1 : v (C-H, C = C) 3114w; V (C = O) 1627S; V (C-BR) 755m. 1 H NMR (700MHz, CDCL 3 δ: 1.35-1.77 (m, 8h), 1.96 (D, J = 11.4 Hz, 2H), 2.60 (DT, J = 6.3, 13.1 Hz, 2H,), 2.78 (DT, J = 11.8, 21.7 Hz, 4H), 2.88 (D, J = 5.4 Hz, 2H), 3.05 (DD, J = 3.9, 12.8 Hz, 1H), 3.84 (D, J = 5.9 Hz, 2H), 4.34 (DT, J = 5.1, 12.7 Hz, 1H), 4.64 (Tt, J = 3.1, 9.1 Hz, 1H), 6.27 (T, J = 2.4 Hz, 1H), 7.32 (D, J = 2.5 Hz, 1H). 13 C NMR (176MHz, CDCL 3 δ: 20.42, 20.94, 26.44, 27.52, 31.09, 35.49, 37.15, 41.85, 42.11, 50.0, 53.37 (2C), 57.01, 63.57, 108.61, 125.84, 129.22, 165.52.LRMS (ESI) m / z: Calcd 392.12 , Observed 393.6 [M + H] + .
[0071] Figure 1 ~ 5 The infrared spectrum of 3-Cl-Pyr-Mat, mass spectrum spectrum, hydrogen spectrum, carbon spectrum and crystal structure and cell chart. Its crystallographic data and structural analysis parameters are shown in Table 1.
[0072] Table 1 3-Cl-Pyr-MAT crystallographic data...
Embodiment 2
[0075] Example 2, 3-br-Pyr-Mat spectrum data:
[0076] SELECTED IR DATA (KBR Disk, CM -1 : υ (C-H, C = C) 3109W; V (C = O) 1622S; V (C-I) 759m. 1 H NMR (700MHz, CDCL 3 δ: 1.36-1.62 (m, 4H), 1.65-1.78 (m, 4H), 1.86-1.98 (m, 2H), 2.60 (DDT, J = 5.8, 8.3, 14.0 Hz, 1H), 2.74-2.81 (m, 4H), 2.88 (D, J = 1.8 Hz, 2H), 3.05 (T, J = 12.6Hz, 1H), 3.83 (Q, J = 5.4 Hz, 2H,) 4.33 (TD, J = 4.5, 12.7 Hz, 1H), 4.70 (TT, J = 3.0, 8.7 Hz, 1 H), 6.41 (T, J = 2.4 Hz, 1H), 7.26 (D, J = 2.4 Hz 1h). 13 C NMR (176MHz, CDCL 3 Δ: 20.76, 21.28, 26.79, 27.87, 31.53, 35.43, 37.55, 42.20, 42.43, 50.55, 53.62 (2C), 57.36, 63.90, 95.93.17, 129.55, 165.93.LRMS (ESI) m / z: Calcd 440.11 , Observed 441.4 [M + H] + .
[0077] Figure 6 ~ 10 The infrared spectrum of 3-Br-Pyr-Mat, mass spectrum spectrum, hydrogen spectrum, carbon spectrum and crystal structure and cell chart. Its crystallographic data and its structural analysis parameters are shown in Table 2.
[0078] Table 2 3-Br-Pyr-Mat crystallographic data and st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com