Application of carboxamide triazole compound or salt thereof as sphingosine kinase 2 inhibitor
A kind of carboxamine triazoles, sphingosine kinase technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
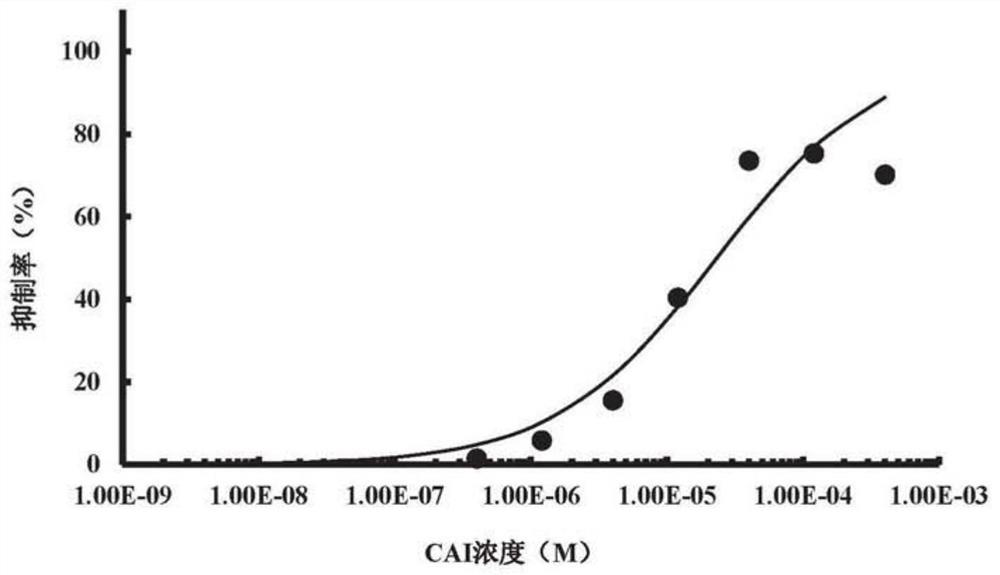
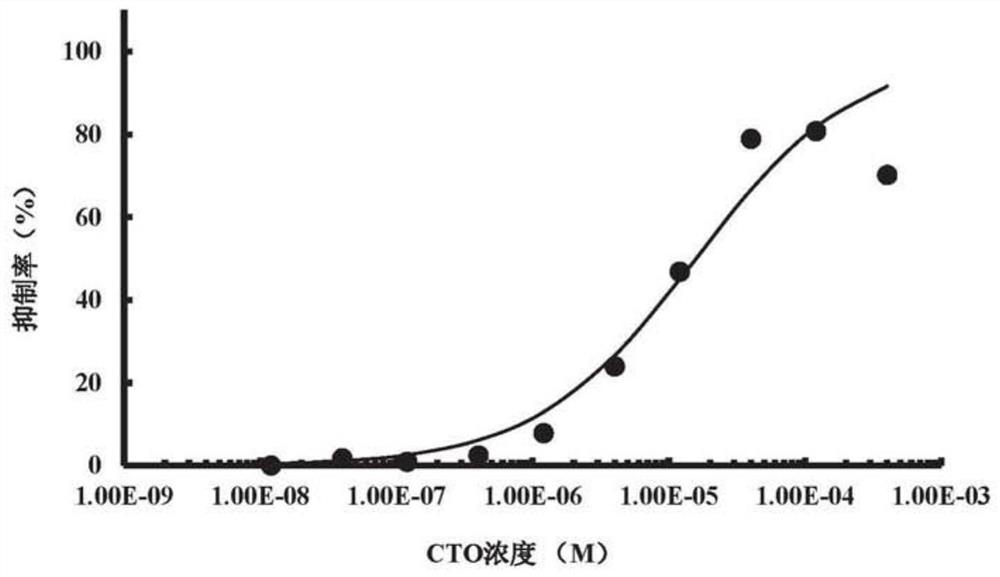
[0077] This embodiment is used to illustrate the half-inhibitory concentration (IC) of CAI and CTO for the inhibitory effect of SPHK2 enzyme activity 50 )
[0078] The inhibition of SPHK2 enzyme activity was detected by Off-chip Mobility Shift Assay:
[0079] ①. The compounds to be tested (CAI, CTO and ABC294640) were dissolved in dimethyl sulfoxide (DMSO) to prepare a solution whose concentration was 100 times the final concentration of the test. The solution was then diluted 25 times with assay buffer (20 mM HEPES, 0.01% Triton X-100, 1 mM DTT, pH 7.5) to obtain a 4-fold concentration compound solution (4×compound solution). In a similar manner, a 4x positive control solution was prepared using the positive control (PF-543).
[0080] ②. Use reagent buffer (20mM HEPES, 0.01% Triton X-100, 5mM DTT, pH 7.5) to configure 4× substrate / ATP / metal solution (see Table 1 for the concentration of substrate, ATP and metal), and use assay buffer Make up 2× kinase solution.
[0081] C...
Embodiment 2
[0094] This example is used to illustrate that CAI and CTO can selectively inhibit the enzymatic activity of SPHK2.
[0095] SPHK includes two subtypes, SPHK1 and SPHK2. In order to clarify the selectivity of CAI and CTO for the inhibition of SPHK2 enzyme activity, the inhibition rate (%) of CAI and CTO on SPHK1 and SPHK2 enzyme activity at the concentration of 40 μM was detected respectively. The method for detecting the enzyme inhibitory activity of SPHK2 is the same as that in Example 1. When detecting the enzyme activity inhibition rate (%) to SPHK1, replace SPHK2 kinase with SPHK1 kinase, the concentration of each component in the substrate / ATP / metal solution is shown in Table 3, and other operations are with the enzyme inhibition of SPHK2 among the embodiment 1 Activity detection method.
[0096] Concentration of each component in the substrate / ATP / metal solution in table 3
[0097]
[0098] The results showed that 40 μM CAI inhibited the enzyme activities of SPHK1...
Embodiment 3
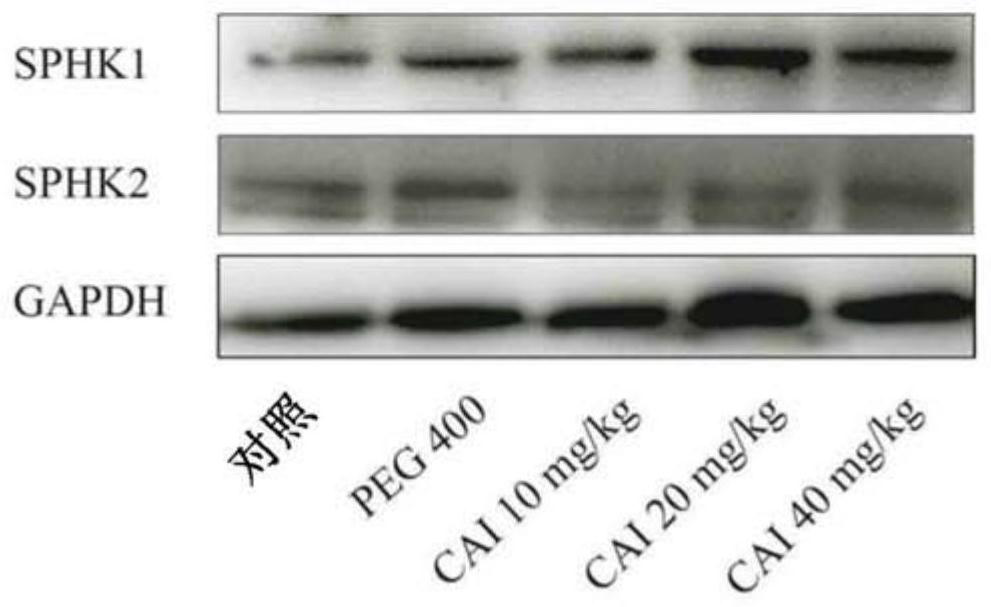
[0100] This example is used to illustrate that CAI's inhibitory effect on SPHK2 enzyme activity is not achieved by affecting its expression.
[0101] In order to clarify whether the inhibitory effect of CAI on SPHK2 enzyme activity is achieved by affecting its expression, rat collagen-induced arthritis (collagen-induced arthritis, CIA) was established, and the protein expression levels of SPHK1 and SPHK2 in rat joint synovium were detected:
[0102] ①. Preparation of rat CIA model: Dissolve bovine type II collagen (CII) in 50 mM acetic acid, stir at 4°C to fully dissolve it, the concentration is 4 mg / mL, put it in a refrigerator at 4°C overnight, and mix with Mix equal volumes of complete Freund's adjuvant containing 4 mg / mL inactivated BCG, fully emulsify in ice bath, and make CⅡ emulsion. On the 0th day (d0), except the control group, other groups were intradermally injected with 0.2 mL emulsion at the base of the tail and back of each rat. On d7, except for the control gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com